The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation
- PMID: 33061891
- PMCID: PMC7518314
- DOI: 10.3389/fncel.2020.00274
The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation
Abstract
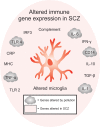
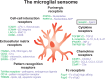
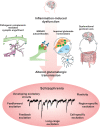
Schizophrenia is a disorder with a heterogeneous etiology involving complex interplay between genetic and environmental risk factors. The immune system is now known to play vital roles in nervous system function and pathology through regulating neuronal and glial development, synaptic plasticity, and behavior. In this regard, the immune system is positioned as a common link between the seemingly diverse genetic and environmental risk factors for schizophrenia. Synthesizing information about how the immune-brain axis is affected by multiple factors and how these factors might interact in schizophrenia is necessary to better understand the pathogenesis of this disease. Such knowledge will aid in the development of more translatable animal models that may lead to effective therapeutic interventions. Here, we provide an overview of the genetic risk factors for schizophrenia that modulate immune function. We also explore environmental factors for schizophrenia including exposure to pollution, gut dysbiosis, maternal immune activation and early-life stress, and how the consequences of these risk factors are linked to microglial function and dysfunction. We also propose that morphological and signaling deficits of the blood-brain barrier, as observed in some individuals with schizophrenia, can act as a gateway between peripheral and central nervous system inflammation, thus affecting microglia in their essential functions. Finally, we describe the diverse roles that microglia play in response to neuroinflammation and their impact on brain development and homeostasis, as well as schizophrenia pathophysiology.
Keywords: Environment; brain development; genes; microglia; neurodevelopmental; neuroinflammation; risk factors; schizophrenia.
Copyright © 2020 Comer, Carrier, Tremblay and Cruz-Martín.
Figures

Similar articles
-
Emerging epigenetic dynamics in gut-microglia brain axis: experimental and clinical implications for accelerated brain aging in schizophrenia.Front Cell Neurosci. 2023 May 15;17:1139357. doi: 10.3389/fncel.2023.1139357. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37256150 Free PMC article. Review.
-
Microglial Activation and Psychotic Disorders: Evidence from Pre-clinical and Clinical Studies.Curr Top Behav Neurosci. 2020;44:161-205. doi: 10.1007/7854_2018_81. Curr Top Behav Neurosci. 2020. PMID: 30828767 Review.
-
Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis.Biomed Pharmacother. 2023 Apr;160:114308. doi: 10.1016/j.biopha.2023.114308. Epub 2023 Jan 28. Biomed Pharmacother. 2023. PMID: 36709599
-
Microglia-neuron interactions in schizophrenia.Front Cell Neurosci. 2024 Mar 6;18:1345349. doi: 10.3389/fncel.2024.1345349. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38510107 Free PMC article. Review.
-
Techniques and Methods of Animal Brain Surgery: Perfusion, Brain Removal, and Histological Techniques.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 15. PMID: 26269921 Free Books & Documents. Review.
Cited by
-
Synapse-specific roles for microglia in development: New horizons in the prefrontal cortex.Front Mol Neurosci. 2022 Aug 8;15:965756. doi: 10.3389/fnmol.2022.965756. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36003220 Free PMC article. Review.
-
Progress and Pitfalls in Developing Agents to Treat Neurocognitive Deficits Associated with Schizophrenia.CNS Drugs. 2022 Aug;36(8):819-858. doi: 10.1007/s40263-022-00935-z. Epub 2022 Jul 13. CNS Drugs. 2022. PMID: 35831706 Free PMC article. Review.
-
Brain microvascular endothelial cells and blood-brain barrier dysfunction in psychotic disorders.Mol Psychiatry. 2023 Sep;28(9):3698-3708. doi: 10.1038/s41380-023-02255-0. Epub 2023 Sep 20. Mol Psychiatry. 2023. PMID: 37730841
-
Repetitive Transcranial Magnetic Stimulation: A Potential Treatment for Obesity in Patients with Schizophrenia.Behav Sci (Basel). 2021 Jun 11;11(6):86. doi: 10.3390/bs11060086. Behav Sci (Basel). 2021. PMID: 34208079 Free PMC article. Review.
-
Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders.Curr Opin Psychiatry. 2021 May 1;34(3):222-227. doi: 10.1097/YCO.0000000000000696. Curr Opin Psychiatry. 2021. PMID: 33560023 Free PMC article. Review.
References
-
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37 13–25. - PubMed
-
- Allen J. L., Liu X., Weston D., Prince L., Oberdörster G., Finkelstein J. N., et al. (2014). Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol. Sci. 140 160–178. 10.1093/toxsci/kfu059 - DOI - PMC - PubMed
-
- Allen J. L., Oberdorster G., Wong C., Klocke C., Sobolewski M., Conrad K. (2017). Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 59 140–154. 10.1016/j.neuro.2015.12.014 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

