Magnesium Sulfate Improves Some Risk Factors for Atherosclerosis in Patients Suffering from One or Two Coronary Artery Diseases: A Double-blind Clinical Trial Study
- PMID: 33061673
- PMCID: PMC7524176
- DOI: 10.2147/CPAA.S261264
Magnesium Sulfate Improves Some Risk Factors for Atherosclerosis in Patients Suffering from One or Two Coronary Artery Diseases: A Double-blind Clinical Trial Study
Abstract
Purpose: Given the beneficial effect of MgSO4 on the cardiovascular system, this study was designed to investigate the effect of MgSO4 administration on suppressing some atherosclerotic risk factors in moderate coronary artery disease patients with one or two atherosclerotic vessels.
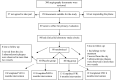
Patients and methods: In a randomized double-blind placebo-controlled clinical trial study, 64 patients with moderate coronary artery disease (55-69% stenosis) were selected according to angiography findings. Patients were divided into four groups including patients with one or two atherosclerotic vessels treated with MgSO4 (Mg-treated-VR1, Mg-treated-VR2, respectively), placebo treated patients with one or two atherosclerotic vessels (Control-VR1, Control-VR2, respectively). The patients received either placebo or MgSO4 supplement capsule containing 300 mg MgSO4 for six months on a daily basis. ESR, Ca/Mg ratio, urine Mg level, serum Mg, fibrinogen, homocysteine, uric acid, Na, K, Ca, CRP, T3, T4, TSH, BUN, and Cr concentrations were measured at baseline and every three months.
Results: Serum T3, Ca, K, homocysteine, CRP, and Mg concentrations were significantly improved in Mg-treated groups compared to placebo groups.
Conclusion: The results of this study showed that despite the slight change in serum magnesium level, oral administration of MgSO4for six months could slightly reduce the serum levels of some inflammatory and vascular factors in moderate coronary artery disease patients.
Keywords: CRP; MgSO4; atherosclerosis; fibrinogen; homocysteine; thyroid hormones.
© 2020 Sobhani et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Magnesium Sulfate Administration in Moderate Coronary Artery Disease Patients Improves Atherosclerotic Risk Factors: A Double-Blind Clinical Trial Study.J Cardiovasc Pharmacol. 2020 Sep;76(3):321-328. doi: 10.1097/FJC.0000000000000874. J Cardiovasc Pharmacol. 2020. PMID: 32618829 Clinical Trial.
-
Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage.J Neurosurg. 2002 Mar;96(3):510-4. doi: 10.3171/jns.2002.96.3.0510. J Neurosurg. 2002. PMID: 11883835 Clinical Trial.
-
Low-dose Magnesium Sulfate Versus High Dose in the Early Management of Rapid Atrial Fibrillation: Randomized Controlled Double-blind Study (LOMAGHI Study).Acad Emerg Med. 2019 Feb;26(2):183-191. doi: 10.1111/acem.13522. Epub 2018 Oct 25. Acad Emerg Med. 2019. PMID: 30025177 Clinical Trial.
-
Inhaled magnesium sulfate in the treatment of acute asthma.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD003898. doi: 10.1002/14651858.CD003898.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003898. doi: 10.1002/14651858.CD003898.pub3. PMID: 15846687 Updated. Review.
-
Inhaled magnesium sulfate in the treatment of acute asthma.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD003898. doi: 10.1002/14651858.CD003898.pub3. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2005 Oct 19;(4):CD003898. doi: 10.1002/14651858.CD003898.pub4. PMID: 16034914 Updated. Review.
Cited by
-
The Role of Txnip in Mediating Low-Magnesium-Driven Endothelial Dysfunction.Int J Mol Sci. 2023 May 6;24(9):8351. doi: 10.3390/ijms24098351. Int J Mol Sci. 2023. PMID: 37176057 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

