IL-6/STAT3 Signaling Contributes to Sorafenib Resistance in Hepatocellular Carcinoma Through Targeting Cancer Stem Cells
- PMID: 33061451
- PMCID: PMC7533247
- DOI: 10.2147/OTT.S262089
IL-6/STAT3 Signaling Contributes to Sorafenib Resistance in Hepatocellular Carcinoma Through Targeting Cancer Stem Cells
Erratum in
-
Erratum: IL-6/STAT3 Signaling Contributes to Sorafenib Resistance in Hepatocellular Carcinoma Through Targeting Cancer Stem Cells [Corrigendum].Onco Targets Ther. 2020 Nov 27;13:12253-12254. doi: 10.2147/OTT.S292577. eCollection 2020. Onco Targets Ther. 2020. PMID: 33273830 Free PMC article.
Retraction in
-
IL-6/STAT3 Signaling Contributes to Sorafenib Resistance in Hepatocellular Carcinoma Through Targeting Cancer Stem Cells [Retraction].Onco Targets Ther. 2022 Aug 22;15:871-872. doi: 10.2147/OTT.S386447. eCollection 2022. Onco Targets Ther. 2022. PMID: 36033902 Free PMC article.
Abstract
Background: Sorafenib is the standard first-line treatment for advanced hepatocellular carcinoma (HCC), even though acquired resistance to sorafenib has been found in many HCC patients, resulting in poor prognosis. Accumulating evidence demonstrates that liver cancer stem cells (LCSCs) contribute to sorafenib resistance in HCC. The inflammatory factor interleukin 6 (IL-6) plays a role in sorafenib resistance in HCC. However, the mechanism by which IL-6 in LCSCs is involved in the process of HCC sorafenib resistance remains elusive.
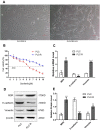
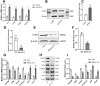
Methods: In this study, the sorafenib-resistant cell line PLC/PRF/5-R was generated by the concentration gradient method, and cell viability was determined by the CCK-8 assay. LCSCs were isolated from the PLC/PRF/5-R cell line by flow cytometry, and tumorigenesis was confirmed in nude mice. Blockade of IL-6 cells was achieved by lentiviral-mediated interference. The protein levels of stem cell markers (EpCAM, CD44), stemness markers (Oct3/4, β-catenin), and hepatocyte differentiation markers (glucose-6-phosphate, AFP) were measured by Western blotting analysis. Finally, a xenograft model was used to evaluate the function of IL-6 in the sorafenib resistance of HCC.
Results: The stable sorafenib-resistant HCC cell line PLC/PRF/5-R was established and showed significant epithelial-mesenchymal transition (EMT) characteristics; the isolated resistant LCSCs from PLC/PRF/5-R were more tumorigenic than the control LCSCs. We showed that IL-6, IL-6R, STAT3 and GP130 expression were dramatically increased in resistant LCSCs compared to control LCSCs. Downregulation of IL-6 expression with short hairpin RNA (shRNA) restored sorafenib sensitivity in resistant LCSCs, suggesting the critical roles of IL-6/STAT3 in inducing sorafenib resistance. Furthermore, a xenograft tumor model showed that IL-6 downregulation improved the antitumor effect of sorafenib.
Conclusion: LCSCs play an important role in sorafenib-resistant HCC, and inhibition of the IL-6/STAT3 signaling pathway improves the antitumor effects of sorafenib against HCC in vitro and in vivo. These findings demonstrate that IL-6 in LCSCs may function as a novel target for combating sorafenib resistance in HCC.
Keywords: IL-6/STAT3 signaling; cancer stem cells; drug resistance; hepatocellular carcinoma; sorafenib.
© 2020 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
DNMT3b/OCT4 expression confers sorafenib resistance and poor prognosis of hepatocellular carcinoma through IL-6/STAT3 regulation.J Exp Clin Cancer Res. 2019 Nov 26;38(1):474. doi: 10.1186/s13046-019-1442-2. J Exp Clin Cancer Res. 2019. PMID: 31771617 Free PMC article.
-
IL-22 signaling promotes sorafenib resistance in hepatocellular carcinoma via STAT3/CD155 signaling axis.Front Immunol. 2024 Mar 25;15:1373321. doi: 10.3389/fimmu.2024.1373321. eCollection 2024. Front Immunol. 2024. PMID: 38596684 Free PMC article.
-
Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway.J Exp Clin Cancer Res. 2019 Dec 30;38(1):505. doi: 10.1186/s13046-019-1508-1. J Exp Clin Cancer Res. 2019. PMID: 31888685 Free PMC article.
-
Targeting liver cancer stem cells for the treatment of hepatocellular carcinoma.Therap Adv Gastroenterol. 2019 Jan 22;12:1756284818821560. doi: 10.1177/1756284818821560. eCollection 2019. Therap Adv Gastroenterol. 2019. PMID: 30719075 Free PMC article. Review.
-
Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies.Cells. 2020 May 26;9(6):1331. doi: 10.3390/cells9061331. Cells. 2020. PMID: 32466488 Free PMC article. Review.
Cited by
-
Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment.Cancer Cell Int. 2022 Feb 11;22(1):73. doi: 10.1186/s12935-021-02435-4. Cancer Cell Int. 2022. PMID: 35148789 Free PMC article. Review.
-
Inhibition of Bone Morphogenetic Protein 2 Suppresses the Stemness Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma via the MAPK/ERK Pathway.Cancer Manag Res. 2021 Jan 27;13:773-785. doi: 10.2147/CMAR.S281969. eCollection 2021. Cancer Manag Res. 2021. PMID: 33536785 Free PMC article.
-
Link of sorafenib resistance with the tumor microenvironment in hepatocellular carcinoma: Mechanistic insights.Front Pharmacol. 2022 Aug 22;13:991052. doi: 10.3389/fphar.2022.991052. eCollection 2022. Front Pharmacol. 2022. PMID: 36071839 Free PMC article. Review.
-
Dual Targeting of Sorafenib-Resistant HCC-Derived Cancer Stem Cells.Curr Oncol. 2021 Jun 11;28(3):2150-2172. doi: 10.3390/curroncol28030200. Curr Oncol. 2021. PMID: 34208001 Free PMC article.
-
Baseline Interleukin-6 and -8 predict response and survival in patients with advanced hepatocellular carcinoma treated with sorafenib monotherapy: an exploratory post hoc analysis of the SORAMIC trial.J Cancer Res Clin Oncol. 2022 Feb;148(2):475-485. doi: 10.1007/s00432-021-03627-1. Epub 2021 Apr 14. J Cancer Res Clin Oncol. 2022. PMID: 33855585 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

