CD40L-stimulated B cells for ex-vivo expansion of polyspecific non-human primate regulatory T cells for translational studies
- PMID: 33058141
- PMCID: PMC7874833
- DOI: 10.1111/cei.13537
CD40L-stimulated B cells for ex-vivo expansion of polyspecific non-human primate regulatory T cells for translational studies
Abstract
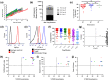
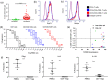
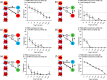
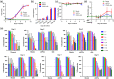
The therapeutic applications of regulatory T cells (Tregs ) include treating autoimmune diseases, graft-versus-host disease and induction of transplantation tolerance. For ex-vivo expanded Tregs to be used in deceased donor transplantation, they must be able to suppress T cell responses to a broad range of human leukocyte antigen (HLA). Here, we present a novel approach for the expansion of polyspecific Tregs in cynomolgus macaques that was adapted from a good manufacturing practice-compliant protocol. Tregs were isolated by fluorescence-activated cell sorting (FACS) and expanded in the presence of a panel of CD40L-stimulated B cells (CD40L-sBc). Prior to Treg culture, CD40L-sBc were expanded in vitro from multiple major histocompatibility complex (MHC)-disparate macaques. Expanded Tregs expressed high levels of forkhead box protein 3 (FoxP3) and Helios, a high percentage of Treg -specific demethylated region (TSDR) demethylation and strong suppression of naïve T cell responses in vitro. In addition, these Tregs produced low levels of inflammatory cytokines and were able to expand post-cryopreservation. Specificity assays confirmed that these Tregs were suppressive upon activation by any antigen-presenting cells (APCs) whose MHC was shared by CD40L-sBc used during expansion, proving that they are polyspecific. We developed an approach for the expansion of highly suppressive cynomolgus macaque polyspecific Tregs through the use of a combination of CD40L-engineered B cells with the potential to be translated to clinical studies. To our knowledge, this is the first report that uses a pool of MHC-mismatched CD40L-sBc to create polyspecific Tregs suitable for use in deceased-donor transplants.
Keywords: CD40L-stimulated B cells; non-human primates; regulatory T cells; tolerance.
© 2020 British Society for Immunology.
Conflict of interest statement
Q. T. is a co‐inventor on two patents on regulatory T cell therapy and a cofounder of Sonoma Biotherapeutics. The rest of the authors declare that they have no competing interests.
Figures


Similar articles
-
Generation and Characterization of Alloantigen-Specific Regulatory T Cells For Clinical Transplant Tolerance.Sci Rep. 2018 Jan 18;8(1):1136. doi: 10.1038/s41598-018-19621-6. Sci Rep. 2018. PMID: 29348660 Free PMC article.
-
A Comparison of Ex Vivo Expanded Human Regulatory T Cells Using Allogeneic Stimulated B Cells or Monocyte-Derived Dendritic Cells.Front Immunol. 2021 Jun 18;12:679675. doi: 10.3389/fimmu.2021.679675. eCollection 2021. Front Immunol. 2021. PMID: 34220826 Free PMC article.
-
Characterization, biology, and expansion of regulatory T cells in the Cynomolgus macaque for preclinical studies.Am J Transplant. 2019 Aug;19(8):2186-2198. doi: 10.1111/ajt.15313. Epub 2019 Mar 29. Am J Transplant. 2019. PMID: 30768842 Free PMC article.
-
Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review.
-
Coexpression of Helios in Foxp3+ Regulatory T Cells and Its Role in Human Disease.Dis Markers. 2021 Jun 22;2021:5574472. doi: 10.1155/2021/5574472. eCollection 2021. Dis Markers. 2021. PMID: 34257746 Free PMC article. Review.
Cited by
-
B cell phenotype, activity, and function in idiopathic nephrotic syndrome.Pediatr Res. 2023 Jun;93(7):1828-1836. doi: 10.1038/s41390-022-02336-w. Epub 2022 Oct 31. Pediatr Res. 2023. PMID: 36316536 Review.
-
Non-human Primate Regulatory T Cells and Their Assessment as Cellular Therapeutics in Preclinical Transplantation Models.Front Cell Dev Biol. 2021 Jun 15;9:666959. doi: 10.3389/fcell.2021.666959. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34211972 Free PMC article. Review.
-
B cell-T cell interplay in immune regulation: A focus on follicular regulatory T and regulatory B cell functions.Front Cell Dev Biol. 2022 Sep 23;10:991840. doi: 10.3389/fcell.2022.991840. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36211467 Free PMC article. Review.
References
-
- Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without. J Immunol 2013; 191:5785–91. - PubMed
-
- Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med 2005; 352:1371–3. - PubMed
-
- Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer 2009; 125:1755–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

