Carnosic acid improves porcine early embryonic development by inhibiting the accumulation of reactive oxygen species
- PMID: 33055461
- PMCID: PMC7768177
- DOI: 10.1262/jrd.2020-086
Carnosic acid improves porcine early embryonic development by inhibiting the accumulation of reactive oxygen species
Abstract
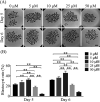
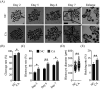
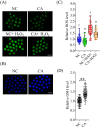
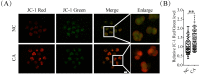
Carnosic acid (CA), a natural catechol rosin diterpene, is used as an additive in animal feeds and human foods. However, the effects of CA on mammalian reproductive processes, especially early embryonic development, are unclear. In this study, we added CA to parthenogenetically activated porcine embryos in an in vitro culture medium to explore the influence of CA on apoptosis, proliferation, blastocyst formation, reactive oxygen species (ROS) levels, glutathione (GSH) levels, mitochondrial membrane potential, and embryonic development-related gene expression. The results showed that supplementation with 10 μM CA during in vitro culture significantly improved the cleavage rates, blastocyst formation rates, hatching rates, and total numbers of cells of parthenogenetically activated porcine embryos compared with no supplementation. More importantly, supplementation with CA also improved GSH levels and mitochondrial membrane potential, reduced natural ROS levels in blastomeres, upregulated Nanog, Sox2, Gata4, Cox2, Itga5, and Rictor expression, and downregulated Birc5 and Caspase3 expression. These results suggest that CA can improve early porcine embryonic development by regulating oxidative stress. This study elucidates the effects of CA on early embryonic development and their potential mechanisms, and provides new applications for improving the quality of in vitro-developed embryos.
Keywords: Antioxidant; Carnosic acid; Embryo development; Oxidative stress; Porcine.
Conflict of interest statement
The authors have no conflicts of interest regarding the contents of this article.
Figures

Similar articles
-
Dihydromyricetin supplementation during in vitro culture improves porcine oocyte developmental competence by regulating oxidative stress.J Reprod Dev. 2023 Feb 8;69(1):10-17. doi: 10.1262/jrd.2022-031. Epub 2022 Nov 19. J Reprod Dev. 2023. PMID: 36403957 Free PMC article.
-
Supplementation with spermine during in vitro maturation of porcine oocytes improves early embryonic development after parthenogenetic activation and somatic cell nuclear transfer.J Anim Sci. 2016 Mar;94(3):963-70. doi: 10.2527/jas.2015-9761. J Anim Sci. 2016. PMID: 27065258
-
Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress.Mol Reprod Dev. 2021 Jan;88(1):55-66. doi: 10.1002/mrd.23440. Epub 2020 Nov 25. Mol Reprod Dev. 2021. PMID: 33241626 Free PMC article.
-
Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress.Theriogenology. 2018 Jul 15;115:38-44. doi: 10.1016/j.theriogenology.2018.04.019. Epub 2018 Apr 23. Theriogenology. 2018. PMID: 29705658
-
α-Ketoglutarate Improves Meiotic Maturation of Porcine Oocytes and Promotes the Development of PA Embryos, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway.Oxid Med Cell Longev. 2022 Feb 21;2022:7113793. doi: 10.1155/2022/7113793. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237383 Free PMC article.
Cited by
-
Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs.J Anim Sci. 2021 Sep 1;99(9):skab237. doi: 10.1093/jas/skab237. J Anim Sci. 2021. PMID: 34370023 Free PMC article.
-
Anethole improves the developmental competence of porcine embryos by reducing oxidative stress via the sonic hedgehog signaling pathway.J Anim Sci Biotechnol. 2023 Feb 22;14(1):32. doi: 10.1186/s40104-022-00824-x. J Anim Sci Biotechnol. 2023. PMID: 36814325 Free PMC article.
-
Dihydromyricetin supplementation during in vitro culture improves porcine oocyte developmental competence by regulating oxidative stress.J Reprod Dev. 2023 Feb 8;69(1):10-17. doi: 10.1262/jrd.2022-031. Epub 2022 Nov 19. J Reprod Dev. 2023. PMID: 36403957 Free PMC article.
-
An Exploration of the Effects of an Early Postpartum Intravenous Infusion with Carnosic Acid on Physiological Responses of Transition Dairy Cows.Antioxidants (Basel). 2021 Sep 16;10(9):1478. doi: 10.3390/antiox10091478. Antioxidants (Basel). 2021. PMID: 34573111 Free PMC article.
References
-
- Opiela J, Samiec M. Characterization of mesenchymal stem cells and their application in experimental embryology. Pol J Vet Sci 2013; 16: 593–599. - PubMed
-
- Callesen MM, Árnadóttir SS, Lyskjaer I, Ørntoft MW, Høyer S, Dagnaes-Hansen F, Liu Y, Li R, Callesen H, Rasmussen MH, Berthelsen MF, Thomsen MK, Schweiger PJ, Jensen KB, Laurberg S, Ørntoft TF, Elverløv-Jakobsen JE, Andersen CL. A genetically inducible porcine model of intestinal cancer. Mol Oncol 2017; 11: 1616–1629. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

