Four-Dimensional Characterization of the Babesia divergens Asexual Life Cycle, from the Trophozoite to the Multiparasite Stage
- PMID: 33055261
- PMCID: PMC7565898
- DOI: 10.1128/mSphere.00928-20
Four-Dimensional Characterization of the Babesia divergens Asexual Life Cycle, from the Trophozoite to the Multiparasite Stage
Abstract
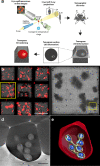
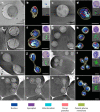
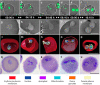
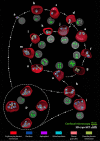
Babesia is an apicomplexan parasite of significance that causes the disease known as babesiosis in domestic and wild animals and in humans worldwide. Babesia infects vertebrate hosts and reproduces asexually by a form of binary fission within erythrocytes/red blood cells (RBCs), yielding a complex pleomorphic population of intraerythrocytic parasites. Seven of them, clearly visible in human RBCs infected with Babesia divergens, are considered the main forms and named single, double, and quadruple trophozoites, paired and double paired pyriforms, tetrad or Maltese Cross, and multiparasite stage. However, these main intraerythrocytic forms coexist with RBCs infected with transient parasite combinations of unclear origin and development. In fact, little is understood about how Babesia builds this complex population during its asexual life cycle. By combining cryo-soft X-ray tomography and video microscopy, main and transitory parasites were characterized in a native whole cellular context and at nanometric resolution. The architecture and kinetics of the parasite population was observed in detail and provide additional data to the previous B. divergens asexual life cycle model that was built on light microscopy. Importantly, the process of multiplication by binary fission, involving budding, was visualized in live parasites for the first time, revealing that fundamental changes in cell shape and continuous rounds of multiplication occur as the parasites go through their asexual multiplication cycle. A four-dimensional asexual life cycle model was built highlighting the origin of several transient morphological forms that, surprisingly, intersperse in a chronological order between one main stage and the next in the cycle.IMPORTANCE Babesiosis is a disease caused by intraerythrocytic Babesia parasites, which possess many clinical features that are similar to those of malaria. This worldwide disease is increasing in frequency and geographical range and has a significant impact on human and animal health. Babesia divergens is one of the species responsible for human and cattle babesiosis causing death unless treated promptly. When B. divergens infects its vertebrate hosts, it reproduces asexually within red blood cells. During its asexual life cycle, B. divergens builds a population of numerous intraerythrocytic (IE) parasites of difficult interpretation. This complex population is largely unexplored, and we have therefore combined three- and four-dimensional imaging techniques to elucidate the origin, architecture, and kinetics of IE parasites. Unveiling the nature of these parasites has provided a vision of the B. divergens asexual cycle in unprecedented detail and is a key step to develop control strategies against babesiosis.
Keywords: Babesia divergens; cryo-soft X-ray tomography; intraerythrocytic asexual cycle; pathogen-host cell interactions; time-lapse video microscopy.
Copyright © 2020 Conesa et al.
Figures

Similar articles
-
Extraordinary high level of propagation of Babesia divergens in severe human babesiosis.Parasitology. 2022 Aug;149(9):1160-1163. doi: 10.1017/S0031182022000439. Epub 2022 May 20. Parasitology. 2022. PMID: 35591780 Free PMC article.
-
Unravelling the cellular and molecular pathogenesis of bovine babesiosis: is the sky the limit?Int J Parasitol. 2019 Feb;49(2):183-197. doi: 10.1016/j.ijpara.2018.11.002. Epub 2019 Jan 26. Int J Parasitol. 2019. PMID: 30690089 Free PMC article. Review.
-
Kinetics of the invasion and egress processes of Babesia divergens, observed by time-lapse video microscopy.Sci Rep. 2018 Sep 20;8(1):14116. doi: 10.1038/s41598-018-32349-7. Sci Rep. 2018. PMID: 30237573 Free PMC article.
-
Morphological comparisons of the bovine piroplasm, Babesia divergens, in cattle and jird (Meriones unguiculatus) erythrocytes.J Parasitol. 1985 Dec;71(6):799-802. J Parasitol. 1985. PMID: 4093812
-
Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance.Clin Microbiol Rev. 2003 Oct;16(4):622-36. doi: 10.1128/CMR.16.4.622-636.2003. Clin Microbiol Rev. 2003. PMID: 14557289 Free PMC article. Review.
Cited by
-
Comparative single-cell transcriptional atlases of Babesia species reveal conserved and species-specific expression profiles.PLoS Biol. 2022 Sep 22;20(9):e3001816. doi: 10.1371/journal.pbio.3001816. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36137068 Free PMC article.
-
Extraordinary high level of propagation of Babesia divergens in severe human babesiosis.Parasitology. 2022 Aug;149(9):1160-1163. doi: 10.1017/S0031182022000439. Epub 2022 May 20. Parasitology. 2022. PMID: 35591780 Free PMC article.
-
Septins and K63 ubiquitin chains are present in separate bacterial microdomains during autophagy of entrapped Shigella.J Cell Sci. 2023 Apr 1;136(7):jcs261139. doi: 10.1242/jcs.261139. Epub 2023 Apr 13. J Cell Sci. 2023. PMID: 36939083 Free PMC article.
-
Imaging of Virus-Infected Cells with Soft X-ray Tomography.Viruses. 2021 Oct 20;13(11):2109. doi: 10.3390/v13112109. Viruses. 2021. PMID: 34834916 Free PMC article. Review.
-
Integration of Genomic and Transcriptomic Data to Elucidate Molecular Processes in Babesia divergens.Methods Mol Biol. 2021;2369:199-215. doi: 10.1007/978-1-0716-1681-9_12. Methods Mol Biol. 2021. PMID: 34313991
