Remdesivir against COVID-19 and Other Viral Diseases
- PMID: 33055231
- PMCID: PMC7566896
- DOI: 10.1128/CMR.00162-20
Remdesivir against COVID-19 and Other Viral Diseases
Abstract
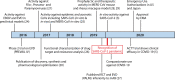
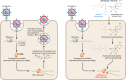
Patients and physicians worldwide are facing tremendous health care hazards that are caused by the ongoing severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) pandemic. Remdesivir (GS-5734) is the first approved treatment for severe coronavirus disease 2019 (COVID-19). It is a novel nucleoside analog with a broad antiviral activity spectrum among RNA viruses, including ebolavirus (EBOV) and the respiratory pathogens Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2. First described in 2016, the drug was derived from an antiviral library of small molecules intended to target emerging pathogenic RNA viruses. In vivo, remdesivir showed therapeutic and prophylactic effects in animal models of EBOV, MERS-CoV, SARS-CoV, and SARS-CoV-2 infection. However, the substance failed in a clinical trial on ebolavirus disease (EVD), where it was inferior to investigational monoclonal antibodies in an interim analysis. As there was no placebo control in this study, no conclusions on its efficacy in EVD can be made. In contrast, data from a placebo-controlled trial show beneficial effects for patients with COVID-19. Remdesivir reduces the time to recovery of hospitalized patients who require supplemental oxygen and may have a positive impact on mortality outcomes while having a favorable safety profile. Although this is an important milestone in the fight against COVID-19, approval of this drug will not be sufficient to solve the public health issues caused by the ongoing pandemic. Further scientific efforts are needed to evaluate the full potential of nucleoside analogs as treatment or prophylaxis of viral respiratory infections and to develop effective antivirals that are orally bioavailable.
Keywords: COVID-19; MERS-CoV; SARS-CoV; SARS-CoV-2; antiviral; coronavirus; ebolavirus; remdesivir.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2.Nature. 2020 Sep;585(7824):273-276. doi: 10.1038/s41586-020-2423-5. Epub 2020 Jun 9. Nature. 2020. PMID: 32516797 Free PMC article.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
-
SARS, MERS and SARS-CoV-2 (COVID-19) treatment: a patent review.Expert Opin Ther Pat. 2020 Aug;30(8):567-579. doi: 10.1080/13543776.2020.1772231. Epub 2020 Jun 7. Expert Opin Ther Pat. 2020. PMID: 32429703 Review.
-
Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19.Pharmacotherapy. 2020 Jul;40(7):659-671. doi: 10.1002/phar.2429. Epub 2020 Jun 28. Pharmacotherapy. 2020. PMID: 32446287 Free PMC article. Review.
-
Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial.Trials. 2020 May 24;21(1):422. doi: 10.1186/s13063-020-04352-9. Trials. 2020. PMID: 32448345 Free PMC article.
Cited by
-
In silico identification of potential inhibitors of vital monkeypox virus proteins from FDA approved drugs.Mol Divers. 2023 Oct;27(5):2169-2184. doi: 10.1007/s11030-022-10550-1. Epub 2022 Nov 4. Mol Divers. 2023. PMID: 36331784 Free PMC article.
-
Remdesivir, Renal Function and Short-Term Clinical Outcomes in Elderly COVID-19 Pneumonia Patients: A Single-Centre Study.Clin Interv Aging. 2021 Jun 3;16:1037-1046. doi: 10.2147/CIA.S313028. eCollection 2021. Clin Interv Aging. 2021. PMID: 34113086 Free PMC article.
-
Anno 2021: Which antivirals for the coming decade?Annu Rep Med Chem. 2021;57:49-107. doi: 10.1016/bs.armc.2021.09.004. Epub 2021 Nov 3. Annu Rep Med Chem. 2021. PMID: 34744210 Free PMC article.
-
COVID-19 and Indirect Liver Injury: A Narrative Synthesis of the Evidence.J Clin Transl Hepatol. 2021 Oct 28;9(5):760-768. doi: 10.14218/JCTH.2020.00140. Epub 2021 Jun 16. J Clin Transl Hepatol. 2021. PMID: 34722191 Free PMC article. Review.
-
Pharmacological Treatment for the Management of COVID 19: A Narrative Review.JNMA J Nepal Med Assoc. 2021 Jul 1;59(238):614-621. doi: 10.31729/jnma.5920. JNMA J Nepal Med Assoc. 2021. PMID: 34508415 Free PMC article. Review.
References
-
- Johns Hopkins University. 2020. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins University, Baltimore, MD. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594.... Accessed 6 July 2020.
-
- Ogando NS, Dalebout TJ, Zevenhoven-Dobbe JC, Limpens RWAL, van der Meer Y, Caly L, Druce J, de Vries JJC, Kikkert M, Bárcena M, Sidorov I, Snijder EJ. 22 June 2020. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J Gen Virol doi:10.1099/jgv.0.001453. - DOI - PMC - PubMed
-
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members . 22 May 2020. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med doi:10.1056/NEJMoa2007764. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

