Chromatin Dynamics in Intestinal Epithelial Homeostasis: A Paradigm of Cell Fate Determination versus Cell Plasticity
- PMID: 33051755
- PMCID: PMC7667136
- DOI: 10.1007/s12015-020-10055-0
Chromatin Dynamics in Intestinal Epithelial Homeostasis: A Paradigm of Cell Fate Determination versus Cell Plasticity
Abstract
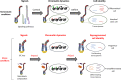
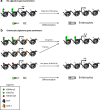
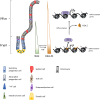
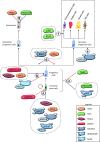
The rapid renewal of intestinal epithelium is mediated by a pool of stem cells, located at the bottom of crypts, giving rise to highly proliferative progenitor cells, which in turn differentiate during their migration along the villus. The equilibrium between renewal and differentiation is critical for establishment and maintenance of tissue homeostasis, and is regulated by signaling pathways (Wnt, Notch, Bmp…) and specific transcription factors (TCF4, CDX2…). Such regulation controls intestinal cell identities by modulating the cellular transcriptome. Recently, chromatin modification and dynamics have been identified as major actors linking signaling pathways and transcriptional regulation in the control of intestinal homeostasis. In this review, we synthesize the many facets of chromatin dynamics involved in controlling intestinal cell fate, such as stemness maintenance, progenitor identity, lineage choice and commitment, and terminal differentiation. In addition, we present recent data underlying the fundamental role of chromatin dynamics in intestinal cell plasticity. Indeed, this plasticity, which includes dedifferentiation processes or the response to environmental cues (like microbiota's presence or food ingestion), is central for the organ's physiology. Finally, we discuss the role of chromatin dynamics in the appearance and treatment of diseases caused by deficiencies in the aforementioned mechanisms, such as gastrointestinal cancer, inflammatory bowel disease or irritable bowel syndrome. Graphical abstract.
Keywords: Chromatin remodeler; Differentiation; Epigenetics; Histone post-translational modification; Homeostasis; Intestinal epithelium; Lineage commitment; Response to environment; Stem cell proliferation.
Conflict of interest statement
Conflict of Interest. The authors declare no conflict of interest.
Figures
Similar articles
-
Control of Intestinal Stemness and Cell Lineage by Histone Variant H2A.Z Isoforms.Mol Cell Biol. 2024;44(11):455-472. doi: 10.1080/10985549.2024.2387720. Epub 2024 Aug 18. Mol Cell Biol. 2024. PMID: 39155414 Free PMC article.
-
Polycomb Repressive Complex 2 Enacts Wnt Signaling in Intestinal Homeostasis and Contributes to the Instigation of Stemness in Diseases Entailing Epithelial Hyperplasia or Neoplasia.Stem Cells. 2017 Feb;35(2):445-457. doi: 10.1002/stem.2479. Epub 2016 Sep 13. Stem Cells. 2017. PMID: 27570105
-
The H2A.Z histone variant integrates Wnt signaling in intestinal epithelial homeostasis.Nat Commun. 2019 Apr 23;10(1):1827. doi: 10.1038/s41467-019-09899-z. Nat Commun. 2019. PMID: 31015444 Free PMC article.
-
Epigenetics in Intestinal Epithelial Cell Renewal.J Cell Physiol. 2016 Nov;231(11):2361-7. doi: 10.1002/jcp.25401. Epub 2016 Apr 26. J Cell Physiol. 2016. PMID: 27061836 Free PMC article. Review.
-
Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision.J Gastroenterol. 2007 Sep;42(9):705-10. doi: 10.1007/s00535-007-2087-z. Epub 2007 Sep 25. J Gastroenterol. 2007. PMID: 17876539 Review.
Cited by
-
Control of Intestinal Stemness and Cell Lineage by Histone Variant H2A.Z Isoforms.Mol Cell Biol. 2024;44(11):455-472. doi: 10.1080/10985549.2024.2387720. Epub 2024 Aug 18. Mol Cell Biol. 2024. PMID: 39155414 Free PMC article.
-
Good Neighbors: The Niche that Fine Tunes Mammalian Intestinal Regeneration.Cold Spring Harb Perspect Biol. 2022 May 27;14(5):a040865. doi: 10.1101/cshperspect.a040865. Cold Spring Harb Perspect Biol. 2022. PMID: 34580119 Free PMC article. Review.
-
Cdx2 Regulates Intestinal EphrinB1 through the Notch Pathway.Genes (Basel). 2021 Jan 28;12(2):188. doi: 10.3390/genes12020188. Genes (Basel). 2021. PMID: 33525395 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

