Murine Epsins Play an Integral Role in Podocyte Function
- PMID: 33051360
- PMCID: PMC7790213
- DOI: 10.1681/ASN.2020050691
Murine Epsins Play an Integral Role in Podocyte Function
Abstract
Background: Epsins, a family of evolutionarily conserved membrane proteins, play an essential role in endocytosis and signaling in podocytes.
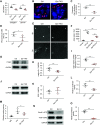
Methods: Podocyte-specific Epn1, Epn2, Epn3 triple-knockout mice were generated to examine downstream regulation of serum response factor (SRF) by cell division control protein 42 homolog (Cdc42).
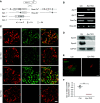
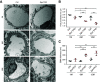
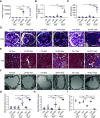
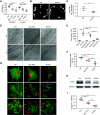
Results: Podocyte-specific loss of epsins resulted in increased albuminuria and foot process effacement. Primary podocytes isolated from these knockout mice exhibited abnormalities in cell adhesion and spreading, which may be attributed to reduced activation of cell division control protein Cdc42 and SRF, resulting in diminished β1 integrin expression. In addition, podocyte-specific loss of Srf resulted in severe albuminuria and foot process effacement, and defects in cell adhesion and spreading, along with decreased β1 integrin expression.
Conclusions: Epsins play an indispensable role in maintaining properly functioning podocytes through the regulation of Cdc42 and SRF-dependent β1 integrin expression.
Keywords: cell adhesion; glomerulosclerosis; podocyte.
Copyright © 2020 by the American Society of Nephrology.
Figures



Similar articles
-
ARHGEF7 (β-PIX) Is Required for the Maintenance of Podocyte Architecture and Glomerular Function.J Am Soc Nephrol. 2020 May;31(5):996-1008. doi: 10.1681/ASN.2019090982. Epub 2020 Mar 18. J Am Soc Nephrol. 2020. PMID: 32188698 Free PMC article.
-
Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis-associated glomerular injury.J Clin Invest. 2014 Apr;124(4):1757-69. doi: 10.1172/JCI67846. Epub 2014 Mar 18. J Clin Invest. 2014. PMID: 24642466 Free PMC article.
-
Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury.Kidney Int. 2013 Nov;84(5):920-30. doi: 10.1038/ki.2013.175. Epub 2013 May 15. Kidney Int. 2013. PMID: 23677246 Free PMC article.
-
Signaling in regulation of podocyte phenotypes.Nephron Physiol. 2009;111(2):p9-15. doi: 10.1159/000191075. Epub 2009 Jan 13. Nephron Physiol. 2009. PMID: 19142027 Free PMC article. Review.
-
Podocyte mitosis - a catastrophe.Curr Mol Med. 2013 Jan;13(1):13-23. doi: 10.2174/1566524011307010013. Curr Mol Med. 2013. PMID: 23176147 Free PMC article. Review.
Cited by
-
Interferon-alpha responsible EPN3 regulates hepatitis B virus replication.Front Med (Lausanne). 2022 Jul 22;9:944489. doi: 10.3389/fmed.2022.944489. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35935763 Free PMC article.
-
Podocyte Endocytosis in Regulating the Glomerular Filtration Barrier.Front Med (Lausanne). 2022 Feb 10;9:801837. doi: 10.3389/fmed.2022.801837. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35223901 Free PMC article. Review.
-
A Novel Model for Nephrotic Syndrome Reveals Associated Dysbiosis of the Gut Microbiome and Extramedullary Hematopoiesis.Cells. 2021 Jun 15;10(6):1509. doi: 10.3390/cells10061509. Cells. 2021. PMID: 34203913 Free PMC article.
References
-
- Nagata M: Podocyte injury and its consequences. Kidney Int 89: 1221–1230, 2016. - PubMed
-
- Martin CE, New LA, Phippen NJ, Keyvani Chahi A, Mitro AE, Takano T, et al. : Multivalent nephrin-Nck interactions define a threshold for clustering and tyrosine-dependent nephrin endocytosis. J Cell Sci 133: jcs236877, 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

