Emergence of Cancer-Associated Fibroblasts as an Indispensable Cellular Player in Bone Metastasis Process
- PMID: 33050237
- PMCID: PMC7600711
- DOI: 10.3390/cancers12102896
Emergence of Cancer-Associated Fibroblasts as an Indispensable Cellular Player in Bone Metastasis Process
Abstract
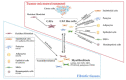
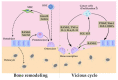
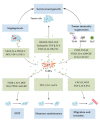
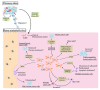
Bone metastasis is frequently complicated in patients with advanced solid cancers such as breast, prostate and lung cancers, and impairs patients' quality of life and prognosis. At the first step of bone metastasis, cancer cells adhere to the endothelium in bone marrow and survive in a dormant state by utilizing hematopoietic niches present therein. Once a dormant stage is disturbed, cancer cells grow through the interaction with various bone marrow resident cells, particularly osteoclasts and osteoblasts. Consequently, osteoclast activation is a hallmark of bone metastasis. As a consequence, the drugs targeting osteoclast activation are frequently used to treat bone metastasis but are not effective to inhibit cancer cell growth in bone marrow. Thus, additional types of resident cells are presumed to contribute to cancer cell growth in bone metastasis sites. Cancer-associated fibroblasts (CAFs) are fibroblasts that accumulate in cancer tissues and can have diverse roles in cancer progression and metastasis. Given the presence of CAFs in bone metastasis sites, CAFs are emerging as an important cellular player in bone metastasis. Hence, in this review, we will discuss the potential roles of CAFs in tumor progression, particularly bone metastasis.
Keywords: bone metastasis; cancer-associated fibroblast; fibroblast; mesenchymal stem cell; myofibroblast.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
Similar articles
-
Carcinoma-associated fibroblasts provide operational flexibility in metastasis.Semin Cancer Biol. 2014 Apr;25:33-46. doi: 10.1016/j.semcancer.2013.12.009. Epub 2014 Jan 7. Semin Cancer Biol. 2014. PMID: 24406210 Review.
-
The Endosteal Niche in Breast Cancer Bone Metastasis.Front Oncol. 2020 Mar 13;10:335. doi: 10.3389/fonc.2020.00335. eCollection 2020. Front Oncol. 2020. PMID: 32232008 Free PMC article. Review.
-
Bone marrow mesenchymal stem cells in microenvironment transform into cancer-associated fibroblasts to promote the progression of B-cell acute lymphoblastic leukemia.Biomed Pharmacother. 2020 Oct;130:110610. doi: 10.1016/j.biopha.2020.110610. Epub 2020 Aug 12. Biomed Pharmacother. 2020. PMID: 34321159
-
Hematopoietic stem cell derived carcinoma-associated fibroblasts: a novel origin.Int J Clin Exp Pathol. 2012;5(9):863-73. Epub 2012 Oct 20. Int J Clin Exp Pathol. 2012. PMID: 23119103 Free PMC article. Review.
-
Musashi-2 in cancer-associated fibroblasts promotes non-small cell lung cancer metastasis through paracrine IL-6-driven epithelial-mesenchymal transition.Cell Biosci. 2023 Nov 8;13(1):205. doi: 10.1186/s13578-023-01158-5. Cell Biosci. 2023. PMID: 37941042 Free PMC article.
Cited by
-
Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis.Front Cell Dev Biol. 2021 Apr 1;9:639514. doi: 10.3389/fcell.2021.639514. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869189 Free PMC article. Review.
-
Bone Metastasis and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer (NSCLC): Microenvironment and Possible Clinical Implications.Int J Mol Sci. 2022 Jun 20;23(12):6832. doi: 10.3390/ijms23126832. Int J Mol Sci. 2022. PMID: 35743275 Free PMC article. Review.
-
Pirfenidone Reduces Epithelial-Mesenchymal Transition and Spheroid Formation in Breast Carcinoma through Targeting Cancer-Associated Fibroblasts (CAFs).Cancers (Basel). 2021 Oct 13;13(20):5118. doi: 10.3390/cancers13205118. Cancers (Basel). 2021. PMID: 34680267 Free PMC article.
-
An integrated analysis of bulk and single-cell sequencing data reveals that EMP1+/COL3A1+ fibroblasts contribute to the bone metastasis process in breast, prostate, and renal cancers.Front Immunol. 2023 Dec 19;14:1313536. doi: 10.3389/fimmu.2023.1313536. eCollection 2023. Front Immunol. 2023. PMID: 38187400 Free PMC article.
-
Single-cell sequencing analysis reveals the relationship between tumor microenvironment cells and oxidative stress in breast cancer bone metastases.Aging (Albany NY). 2023 Jul 19;15(14):6950-6968. doi: 10.18632/aging.204885. Epub 2023 Jul 19. Aging (Albany NY). 2023. PMID: 37470685 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

