Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer
- PMID: 33043122
- PMCID: PMC7541180
- DOI: 10.1055/a-1221-9879
Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer
Abstract
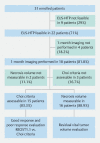
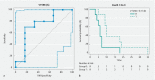
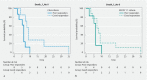
Background and study aims Endoscopic ultrasound (EUS)-guided ablation of pancreatic ductal adenocarcinoma (PDAC) with HybridTherm-Probe (EUS-HTP) is feasible and safe, but the radiological response and ideal tool to measure it have not been investigated yet. The aims of this study were to: 1) assess the radiological response to EUS-HTP evaluating the vital tumor volume reduction rate, Response Evaluation Criteria in Solid Tumors (RECIST1.1) and Choi criteria; 2) determine the prognostic predictive yield of these criteria. Patients and methods A retrospective analysis was performed of patients with locally advanced PDAC after primary treatment or unfit for chemotherapy prospectively treated by EUS-HTP. Computed tomography scan was performed 1 month after EUS-HTP to evaluate: 1) vital tumor volume reduction rate (VTVRR) by measuring necrosis and tumor volumes through a computer-aided detection system; and 2) RECIST1.1 and Choi criteria. Results EUS-HTP was feasible in 22 of 31 patients (71 %), with no severe adverse events. Median post-HTP survival was 7 months (1 - 35). Compared to pre-HTP tumor volume, a significant 1-month VTVRR (mean 21.4 %) was observed after EUS-HTP ( P = 0.005). We identified through ROC analysis a VTVRR > 11.46 % as the best cut-off to determine post-HTP 6-month survival outcome (AUC = 0.733; sensitivity = 70.0 %, specificity = 83.3 %). This cut-off was significantly associated with longer overall survival (HR = 0.372; P = 0.039). According to RECIST1.1 and Choi criteria, good responders to EUS-HTP were 60 % and 46.7 %, respectively. Good responders according to Choi, but not to RECIST1.1, had longer survival (HR = 0.407; P = 0.04). Conclusions EUS-HTP induces a significant 1-month VTVRR. This effect is assessed accurately by evaluation of necrosis and tumor volumes. Use of VTVRR and Choi criteria, but not RECIST 1.1 criteria, might identify patients who could benefit clinically from EUS-HTP.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commecial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Competing interests Prof. M. Enderle and Dr. W. Linzenbold are employees of the research department of Erbe Elektromedizin GmbH, Tubingen, Germany.
Figures
Similar articles
-
EUS-guided ablation with the HybridTherm Probe as second-line treatment in patients with locally advanced pancreatic ductal adenocarcinoma: A case-control study.Endosc Ultrasound. 2022 Sep-Oct;11(5):383-392. doi: 10.4103/EUS-D-21-00200. Endosc Ultrasound. 2022. PMID: 36255026 Free PMC article.
-
Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2023 Jul 21;15(14):3704. doi: 10.3390/cancers15143704. Cancers (Basel). 2023. PMID: 37509365 Free PMC article.
-
Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial.Cancers (Basel). 2021 Sep 8;13(18):4512. doi: 10.3390/cancers13184512. Cancers (Basel). 2021. PMID: 34572743 Free PMC article.
-
Pretherapeutic evaluation of patients with upper gastrointestinal tract cancer using endoscopic and laparoscopic ultrasonography.Dan Med J. 2012 Dec;59(12):B4568. Dan Med J. 2012. PMID: 23290296 Review.
-
Endoscopic ultrasonography-guided tumor ablation.Dig Endosc. 2017 May;29(4):486-494. doi: 10.1111/den.12833. Epub 2017 Mar 16. Dig Endosc. 2017. PMID: 28171697 Review.
Cited by
-
EUS-guided ablation with the HybridTherm Probe as second-line treatment in patients with locally advanced pancreatic ductal adenocarcinoma: A case-control study.Endosc Ultrasound. 2022 Sep-Oct;11(5):383-392. doi: 10.4103/EUS-D-21-00200. Endosc Ultrasound. 2022. PMID: 36255026 Free PMC article.
-
Advancements in Microwave Ablation Techniques for Managing Pancreatic Lesions.Life (Basel). 2023 Nov 4;13(11):2162. doi: 10.3390/life13112162. Life (Basel). 2023. PMID: 38004302 Free PMC article. Review.
-
Immunomodulatory Effects of Endoscopic Ultrasound-Guided Thermal Ablation in Patients with Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2023 Jul 21;15(14):3704. doi: 10.3390/cancers15143704. Cancers (Basel). 2023. PMID: 37509365 Free PMC article.
-
Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial.Cancers (Basel). 2021 Sep 8;13(18):4512. doi: 10.3390/cancers13184512. Cancers (Basel). 2021. PMID: 34572743 Free PMC article.
References
-
- Paiella S, De Pastena M, Romeo F et al.Ablation treatments in unresectable pancreatic cancer. Minerva Chir. 2019;74:263–269. - PubMed
-
- Saccomandi P, Lapergola A, Longo F et al.Thermal ablation of pancreatic cancer: a systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia. 2018;35:398–418. - PubMed
-
- Niu L, Chen J, He L et al.Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. - PubMed
LinkOut - more resources
Full Text Sources

