Cytosolic DNA Sensors and CNS Responses to Viral Pathogens
- PMID: 33042875
- PMCID: PMC7525022
- DOI: 10.3389/fcimb.2020.576263
Cytosolic DNA Sensors and CNS Responses to Viral Pathogens
Abstract
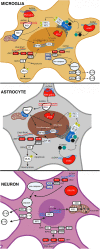
Viral central nervous system (CNS) infections can lead to life threatening encephalitis and long-term neurological deficits in survivors. Resident CNS cell types, such as astrocytes and microglia, are known to produce key inflammatory and antiviral mediators following infection with neurotropic DNA viruses. However, the mechanisms by which glia mediate such responses remain poorly understood. Recently, a class of intracellular pattern recognition receptors (PRRs), collectively known as DNA sensors, have been identified in both leukocytic and non-leukocytic cell types. The ability of such DNA sensors to initiate immune mediator production and contribute to infection resolution in the periphery is increasingly recognized, but our understanding of their role in the CNS remains limited at best. In this review, we describe the evidence for the expression and functionality of DNA sensors in resident brain cells, with a focus on their role in neurotropic virus infections. The available data indicate that glia and neurons can constitutively express, and/or can be induced to express, various disparate DNA sensing molecules previously described in peripheral cell types. Furthermore, multiple lines of investigation suggest that these sensors are functional in resident CNS cells and are required for innate immune responses to viral infections. However, it is less clear whether DNA sensormediated glial responses are beneficial or detrimental, and the answer to this question appears to dependent on the context of the infection with regard to the identity of the pathogen, host cell type, and host species. Defining such parameters will be essential if we are to successfully target these molecules to limit damaging inflammation while allowing beneficial host responses to improve patient outcomes.
Keywords: DNA sensors; astrocytes; microglia; neuroinflammation; viral encephalitis.
Copyright © 2020 Jeffries and Marriott.
Figures
Similar articles
-
Viral CNS infections: role of glial pattern recognition receptors in neuroinflammation.Front Microbiol. 2012 Jun 20;3:201. doi: 10.3389/fmicb.2012.00201. eCollection 2012. Front Microbiol. 2012. PMID: 22723794 Free PMC article.
-
Cytosolic DNA sensors and glial responses to endogenous DNA.Front Immunol. 2023 Mar 14;14:1130172. doi: 10.3389/fimmu.2023.1130172. eCollection 2023. Front Immunol. 2023. PMID: 36999037 Free PMC article. Review.
-
RIG-I is required for VSV-induced cytokine production by murine glia and acts in combination with DAI to initiate responses to HSV-1.Glia. 2015 Dec;63(12):2168-80. doi: 10.1002/glia.22883. Epub 2015 Jul 3. Glia. 2015. PMID: 26146945 Free PMC article.
-
Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis.Glia. 2006 Apr 15;53(6):583-92. doi: 10.1002/glia.20314. Glia. 2006. PMID: 16419089
-
The Interleukin-10 Family of Cytokines and Their Role in the CNS.Front Cell Neurosci. 2018 Nov 27;12:458. doi: 10.3389/fncel.2018.00458. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30542269 Free PMC article. Review.
Cited by
-
Editorial: Host-Pathogen Interaction in the Central Nervous System.Front Cell Infect Microbiol. 2021 Dec 24;11:790761. doi: 10.3389/fcimb.2021.790761. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004356 Free PMC article. No abstract available.
-
Molecular subtypes based on DNA sensors predict prognosis and tumor immunophenotype in hepatocellular carcinoma.Aging (Albany NY). 2023 Jul 14;15(14):6798-6821. doi: 10.18632/aging.204870. Epub 2023 Jul 14. Aging (Albany NY). 2023. PMID: 37451838 Free PMC article.
-
Melatonin Ameliorates Axonal Hypomyelination of Periventricular White Matter by Transforming A1 to A2 Astrocyte via JAK2/STAT3 Pathway in Septic Neonatal Rats.J Inflamm Res. 2021 Nov 12;14:5919-5937. doi: 10.2147/JIR.S337499. eCollection 2021. J Inflamm Res. 2021. PMID: 34803390 Free PMC article.
-
Analysis of Age-Dependent Transcriptomic Changes in Response to Intracerebral Hemorrhage in Mice.Front Mol Neurosci. 2022 May 23;15:908683. doi: 10.3389/fnmol.2022.908683. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35677585 Free PMC article.
-
Impaired STING Activation Due to a Variant in the E3 Ubiquitin Ligase AMFR in a Patient with Severe VZV Infection and Hemophagocytic Lymphohistiocytosis.J Clin Immunol. 2024 Jan 26;44(2):56. doi: 10.1007/s10875-024-01653-5. J Clin Immunol. 2024. PMID: 38277122 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

