lncRNA USP2-AS1 promotes colon cancer progression by modulating Hippo/YAP1 signaling
- PMID: 33042447
- PMCID: PMC7540120
lncRNA USP2-AS1 promotes colon cancer progression by modulating Hippo/YAP1 signaling
Abstract
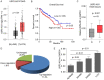
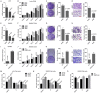
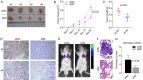
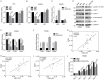
Dysregulation of Hippo signaling by long non-coding RNA (lncRNA) contributes to colon adenocarcinoma (COAD) progression, while the underlying mechanisms remain elusive. Our study shows that lncRNA USP2-AS1 is a Yes-associated protein 1 (YAP1) binding lncRNA, and inactivates Hippo signaling in COAD cells. Moreover, our data indicated that USP2-AS1 lowered the phosph-YAP (S127), elevated the total level of YAP1, and triggered the expression of downstream target genes in COAD cells. The loss- and gain-of function assays demonstrated that USP2-AS1 promotes cellular proliferation and metastasis of COAD cells. Clinically, the USP2-AS1 levels were significantly elevated in COAD tissues and were positively correlated with tumor grade, size, and TNM stage. Collectively, these findings demonstrated that USP2-AS1 modulates and regulates Hippo signaling in COAD and could be a valuable therapeutic target.
Keywords: Hippo signaling; USP2-AS1; cell proliferation; lncRNA; metastasis.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures

Similar articles
-
Hypoxia-Regulated lncRNA USP2-AS1 Drives Head and Neck Squamous Cell Carcinoma Progression.Cells. 2022 Oct 28;11(21):3407. doi: 10.3390/cells11213407. Cells. 2022. PMID: 36359803 Free PMC article.
-
Expression of Hippo pathway genes and their clinical significance in colon adenocarcinoma.Oncol Lett. 2018 Apr;15(4):4926-4936. doi: 10.3892/ol.2018.7911. Epub 2018 Jan 31. Oncol Lett. 2018. PMID: 29541248 Free PMC article.
-
LncRNA ILF3-AS1 Promotes the Progression of Colon Adenocarcinoma Cells Through the miR-619-5p/CAMK1D Axis.Onco Targets Ther. 2021 Mar 12;14:1861-1872. doi: 10.2147/OTT.S296441. eCollection 2021. Onco Targets Ther. 2021. PMID: 33737811 Free PMC article.
-
Overexpression of LncRNA MNX1-AS1/PPFIA4 Activates AKT/HIF-1α Signal Pathway to Promote Stemness of Colorectal Adenocarcinoma Cells.J Oncol. 2022 Oct 3;2022:8303409. doi: 10.1155/2022/8303409. eCollection 2022. J Oncol. 2022. PMID: 36226248 Free PMC article.
-
The role of long non-coding RNA AFAP1-AS1 in human malignant tumors.Pathol Res Pract. 2018 Oct;214(10):1524-1531. doi: 10.1016/j.prp.2018.08.014. Epub 2018 Aug 20. Pathol Res Pract. 2018. PMID: 30173945 Review.
Cited by
-
Crosstalk Among YAP, LncRNA, and Tumor-Associated Macrophages in Tumorigenesis Development.Front Oncol. 2022 Jan 6;11:810893. doi: 10.3389/fonc.2021.810893. eCollection 2021. Front Oncol. 2022. PMID: 35071016 Free PMC article. Review.
-
Hypoxia-Regulated lncRNA USP2-AS1 Drives Head and Neck Squamous Cell Carcinoma Progression.Cells. 2022 Oct 28;11(21):3407. doi: 10.3390/cells11213407. Cells. 2022. PMID: 36359803 Free PMC article.
-
Identification and Characterization of an Ageing-Associated 13-lncRNA Signature That Predicts Prognosis and Immunotherapy in Hepatocellular Carcinoma.J Oncol. 2023 Feb 17;2023:4615297. doi: 10.1155/2023/4615297. eCollection 2023. J Oncol. 2023. PMID: 36844873 Free PMC article.
-
c-Myc-activated USP2-AS1 suppresses senescence and promotes tumor progression via stabilization of E2F1 mRNA.Cell Death Dis. 2021 Oct 27;12(11):1006. doi: 10.1038/s41419-021-04330-2. Cell Death Dis. 2021. PMID: 34707111 Free PMC article.
-
Long noncoding RNAs: functions and mechanisms in colon cancer.Mol Cancer. 2020 Nov 28;19(1):167. doi: 10.1186/s12943-020-01287-2. Mol Cancer. 2020. PMID: 33246471 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
