Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis
- PMID: 33036430
- PMCID: PMC7600908
- DOI: 10.3390/nu12103062
Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis
Abstract
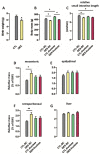
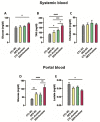
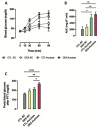
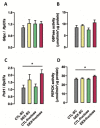
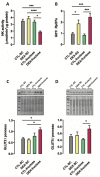
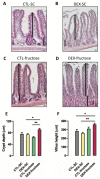
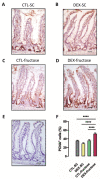
Fructose consumption by rodents modulates both hepatic and intestinal lipid metabolism and gluconeogenesis. We have previously demonstrated that in utero exposure to dexamethasone (DEX) interacts with fructose consumption during adult life to exacerbate hepatic steatosis in rats. The aim of this study was to clarify if adult rats born to DEX-treated mothers would display differences in intestinal gluconeogenesis after excessive fructose intake. To address this issue, female Wistar rats were treated with DEX during pregnancy and control (CTL) mothers were kept untreated. Adult offspring born to CTL and DEX-treated mothers were assigned to receive either tap water (Control-Standard Chow (CTL-SC) and Dexamethasone-Standard Chow (DEX-SC)) or 10% fructose in the drinking water (CTL-fructose and DEX-fructose). Fructose consumption lasted for 80 days. All rats were subjected to a 40 h fasting before sample collection. We found that DEX-fructose rats have increased glucose and reduced lactate in the portal blood. Jejunum samples of DEX-fructose rats have enhanced phosphoenolpyruvate carboxykinase (PEPCK) expression and activity, higher facilitated glucose transporter member 2 (GLUT2) and facilitated glucose transporter member 5 (GLUT5) content, and increased villous height, crypt depth, and proliferating cell nuclear antigen (PCNA) staining. The current data reveal that rats born to DEX-treated mothers that consume fructose during adult life have increased intestinal gluconeogenesis while recapitulating metabolic and morphological features of the neonatal jejunum phenotype.
Keywords: dexamethasone; fructose; intestinal gluconeogenesis; intrauterine growth restriction (IUGR).
Conflict of interest statement
The authors declare no conflicts of interest, financial or otherwise, associated with this article. The authors are responsible for the writing and content of the article.
Figures
Similar articles
-
In Utero Dexamethasone Exposure Exacerbates Hepatic Steatosis in Rats That Consume Fructose During Adulthood.Nutrients. 2019 Sep 5;11(9):2114. doi: 10.3390/nu11092114. Nutrients. 2019. PMID: 31491968 Free PMC article.
-
Dexamethasone programs lower fatty acid absorption and reduced PPAR-γ and fat/CD36 expression in the jejunum of the adult rat offspring.Life Sci. 2021 Jan 15;265:118765. doi: 10.1016/j.lfs.2020.118765. Epub 2020 Nov 13. Life Sci. 2021. PMID: 33189820
-
Maternal dexamethasone and GLP-2 have early effects on intestinal sugar transport in their suckling rat offspring.J Nutr Biochem. 2009 Oct;20(10):771-82. doi: 10.1016/j.jnutbio.2008.07.006. Epub 2008 Nov 6. J Nutr Biochem. 2009. PMID: 18993047
-
The role of fructose transporters in diseases linked to excessive fructose intake.J Physiol. 2013 Jan 15;591(2):401-14. doi: 10.1113/jphysiol.2011.215731. Epub 2012 Nov 5. J Physiol. 2013. PMID: 23129794 Free PMC article. Review.
-
Fructose, pregnancy and later life impacts.Clin Exp Pharmacol Physiol. 2013 Nov;40(11):824-37. doi: 10.1111/1440-1681.12162. Clin Exp Pharmacol Physiol. 2013. PMID: 24033459 Review.
Cited by
-
Low Birth Weight Intensifies Changes in Markers of Hepatocarcinogenesis Induced by Fructose Consumption in Rats.Metabolites. 2022 Sep 21;12(10):886. doi: 10.3390/metabo12100886. Metabolites. 2022. PMID: 36295788 Free PMC article.
-
Dietary Plant-Origin Bio-Active Compounds, Intestinal Functionality, and Microbiome.Nutrients. 2020 Oct 22;12(11):3223. doi: 10.3390/nu12113223. Nutrients. 2020. PMID: 33105549 Free PMC article.
References
-
- Faria J.A., de Araújo T.M., Razolli D.S., Ignácio-Souza L.M., Souza D.N., Bordin S., Anhê G.F. Metabolic impact of light phase-restricted fructose consumption is linked to changes in hypothalamic AMPK phosphorylation and melatonin production in rats. Nutrients. 2017;9:332. doi: 10.3390/nu9040332. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

