The Contractile Phenotype of Skeletal Muscle in TRPV1 Knockout Mice is Gender-Specific and Exercise-Dependent
- PMID: 33036239
- PMCID: PMC7600525
- DOI: 10.3390/life10100233
The Contractile Phenotype of Skeletal Muscle in TRPV1 Knockout Mice is Gender-Specific and Exercise-Dependent
Abstract
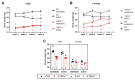
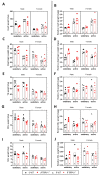
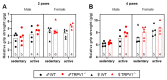
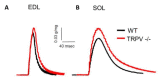
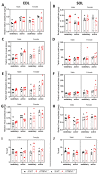
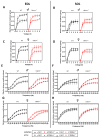
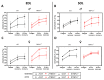
The transient receptor potential vanilloid 1 (TRPV1) belongs to the transient receptor potential superfamily of sensory receptors. TRPV1 is a non-selective cation channel permeable to Ca2+ that is capable of detecting noxious heat temperature and acidosis. In skeletal muscles, TRPV1 operates as a reticular Ca2+-leak channel and several TRPV1 mutations have been associated with two muscle disorders: malignant hyperthermia (MH) and exertional heat stroke (EHS). Although TRPV1-/- mice have been available since the 2000s, TRPV1's role in muscle physiology has not been thoroughly studied. Therefore, the focus of this work was to characterize the contractile phenotype of skeletal muscles of TRPV1-deficient mice at rest and after four weeks of exercise. As MS and EHS have a higher incidence in men than in women, we also investigated sex-related phenotype differences. Our results indicated that, without exercise, TRPV1-/- mice improved in vivo muscle strength with an impairment of skeletal muscle in vitro twitch features, i.e., delayed contraction and relaxation. Additionally, exercise appeared detrimental to TRPV1-/- slow-twitch muscles, especially in female animals.
Keywords: TRPV1; exercise; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures
Similar articles
-
TRPV1 variants impair intracellular Ca2+ signaling and may confer susceptibility to malignant hyperthermia.Genet Med. 2019 Feb;21(2):441-450. doi: 10.1038/s41436-018-0066-9. Epub 2018 Jun 21. Genet Med. 2019. PMID: 29930394 Free PMC article.
-
Accumulation of intramyocyte TRPV1-mediated calcium during heat stress is inhibited by concomitant muscle contractions.J Appl Physiol (1985). 2019 Mar 1;126(3):691-698. doi: 10.1152/japplphysiol.00668.2018. Epub 2019 Jan 24. J Appl Physiol (1985). 2019. PMID: 30676872
-
Calsequestrin-1: a new candidate gene for malignant hyperthermia and exertional/environmental heat stroke.J Physiol. 2009 Jul 1;587(Pt 13):3095-100. doi: 10.1113/jphysiol.2009.171967. Epub 2009 May 5. J Physiol. 2009. PMID: 19417098 Free PMC article. Review.
-
Overlapping Mechanisms of Exertional Heat Stroke and Malignant Hyperthermia: Evidence vs. Conjecture.Sports Med. 2020 Sep;50(9):1581-1592. doi: 10.1007/s40279-020-01318-4. Sports Med. 2020. PMID: 32632746 Review.
-
TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice.Cell Res. 2012 Mar;22(3):551-64. doi: 10.1038/cr.2011.205. Epub 2011 Dec 20. Cell Res. 2012. PMID: 22184011 Free PMC article.
Cited by
-
Identification of nicotinamide N-methyltransferase as a promising therapeutic target for sarcopenia.Aging Cell. 2024 Sep;23(9):e14236. doi: 10.1111/acel.14236. Epub 2024 Jun 5. Aging Cell. 2024. PMID: 38838088 Free PMC article.
-
Thermal escape box: A cost-benefit evaluation paradigm for investigating thermosensation and thermal pain.Neurobiol Pain. 2024 Apr 1;15:100155. doi: 10.1016/j.ynpai.2024.100155. eCollection 2024 Jan-Jun. Neurobiol Pain. 2024. PMID: 38617105 Free PMC article.
-
Involvement of the Transient Receptor Channels in Preclinical Models of Musculoskeletal Pain.Curr Neuropharmacol. 2024;22(1):72-87. doi: 10.2174/1570159X21666230908094159. Curr Neuropharmacol. 2024. PMID: 37694792 Free PMC article. Review.
-
Lingual innervation in male and female marmosets.Neurobiol Pain. 2023 May 31;14:100134. doi: 10.1016/j.ynpai.2023.100134. eCollection 2023 Aug-Dec. Neurobiol Pain. 2023. PMID: 38099285 Free PMC article.
-
Contractile force assessment methods for in vitro skeletal muscle tissues.Elife. 2022 May 23;11:e77204. doi: 10.7554/eLife.77204. Elife. 2022. PMID: 35604384 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

