Rebooting the Epigenomes during Mammalian Early Embryogenesis
- PMID: 33035464
- PMCID: PMC7724468
- DOI: 10.1016/j.stemcr.2020.09.005
Rebooting the Epigenomes during Mammalian Early Embryogenesis
Abstract
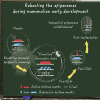
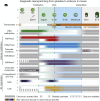
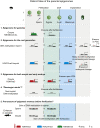
Upon fertilization, terminally differentiated gametes are transformed to a totipotent zygote, which gives rise to an embryo. How parental epigenetic memories are inherited and reprogrammed to accommodate parental-to-zygotic transition remains a fundamental question in developmental biology, epigenetics, and stem cell biology. With the rapid advancement of ultra-sensitive or single-cell epigenome analysis methods, unusual principles of epigenetic reprogramming begin to be unveiled. Emerging data reveal that in many species, the parental epigenome undergoes dramatic reprogramming followed by subsequent re-establishment of the embryo epigenome, leading to epigenetic "rebooting." Here, we discuss recent progress in understanding epigenetic reprogramming and their functions during mammalian early development. We also highlight the conserved and species-specific principles underlying diverse regulation of the epigenome in early embryos during evolution.
Keywords: early embryogenesis; epigenetic reprogramming; epigenetics; epigenome; mammalian development.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Epigenetic Reprogramming in Early Animal Development.Cold Spring Harb Perspect Biol. 2022 Jun 14;14(6):a039677. doi: 10.1101/cshperspect.a039677. Cold Spring Harb Perspect Biol. 2022. PMID: 34400552 Free PMC article. Review.
-
Epigenome in Early Mammalian Development: Inheritance, Reprogramming and Establishment.Trends Cell Biol. 2018 Mar;28(3):237-253. doi: 10.1016/j.tcb.2017.10.008. Epub 2017 Dec 5. Trends Cell Biol. 2018. PMID: 29217127 Review.
-
On Mammalian Totipotency: What Is the Molecular Underpinning for the Totipotency of Zygote?Stem Cells Dev. 2019 Jul 15;28(14):897-906. doi: 10.1089/scd.2019.0057. Stem Cells Dev. 2019. PMID: 31122174 Free PMC article. Review.
-
Insights into epigenetic patterns in mammalian early embryos.Protein Cell. 2021 Jan;12(1):7-28. doi: 10.1007/s13238-020-00757-z. Epub 2020 Jul 15. Protein Cell. 2021. PMID: 32671792 Free PMC article. Review.
-
Consequences of assisted reproductive techniques on the embryonic epigenome in cattle.Reprod Fertil Dev. 2019 Jan;32(2):65-81. doi: 10.1071/RD19276. Reprod Fertil Dev. 2019. PMID: 32188559 Review.
Cited by
-
Reorganization of lamina-associated domains in early mouse embryos is regulated by RNA polymerase II activity.Genes Dev. 2023 Oct 1;37(19-20):901-912. doi: 10.1101/gad.350799.123. Epub 2023 Nov 1. Genes Dev. 2023. PMID: 37914351 Free PMC article.
-
Inter- and transgenerational heritability of preconception chronic stress or alcohol exposure: Translational outcomes in brain and behavior.Neurobiol Stress. 2023 Dec 25;29:100603. doi: 10.1016/j.ynstr.2023.100603. eCollection 2024 Mar. Neurobiol Stress. 2023. PMID: 38234394 Free PMC article.
-
Evolutionary epigenomic analyses in mammalian early embryos reveal species-specific innovations and conserved principles of imprinting.Sci Adv. 2021 Nov 26;7(48):eabi6178. doi: 10.1126/sciadv.abi6178. Epub 2021 Nov 24. Sci Adv. 2021. PMID: 34818044 Free PMC article.
-
Metabolic and epigenetic dysfunctions underlie the arrest of in vitro fertilized human embryos in a senescent-like state.PLoS Biol. 2022 Jun 30;20(6):e3001682. doi: 10.1371/journal.pbio.3001682. eCollection 2022 Jun. PLoS Biol. 2022. PMID: 35771762 Free PMC article.
-
SAMe, Choline, and Valproic Acid as Possible Epigenetic Drugs: Their Effects in Pregnancy with a Special Emphasis on Animal Studies.Pharmaceuticals (Basel). 2022 Feb 3;15(2):192. doi: 10.3390/ph15020192. Pharmaceuticals (Basel). 2022. PMID: 35215304 Free PMC article. Review.
References
-
- Abe K., Inoue A., Suzuki M.G., Aoki F. Global gene silencing is caused by the dissociation of RNA polymerase II from DNA in mouse oocytes. J. Reprod. Dev. 2010;56:502–507. - PubMed
-
- Ai S., Xiong H., Li C.C., Luo Y., Shi Q., Liu Y., Yu X., Li C., He A. Profiling chromatin states using single-cell itChIP-seq. Nat. Cell Biol. 2019;21:1164–1172. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

