Optimization of Eliglustat-Based Glucosylceramide Synthase Inhibitors as Substrate Reduction Therapy for Gaucher Disease Type 3
- PMID: 33035424
- PMCID: PMC7919060
- DOI: 10.1021/acschemneuro.0c00558
Optimization of Eliglustat-Based Glucosylceramide Synthase Inhibitors as Substrate Reduction Therapy for Gaucher Disease Type 3
Abstract
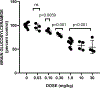
There remain no approved therapies for rare but devastating neuronopathic glyocosphingolipid storage diseases, such as Sandhoff, Tay-Sachs, and Gaucher disease type 3. We previously reported initial optimization of the scaffold of eliglustat, an approved therapy for the peripheral symptoms of Gaucher disease type 1, to afford 2, which effected modest reductions in brain glucosylceramide (GlcCer) in normal mice at 60 mg/kg. The relatively poor pharmacokinetic properties and high Pgp-mediated efflux of 2 prompted further optimization of the scaffold. With a general objective of reducing topological polar surface area, and guided by multiple metabolite identification studies, we were successful at identifying 17 (CCG-222628), which achieves remarkably greater brain exposure in mice than 2. After demonstrating an over 60-fold improvement in potency over 2 at reducing brain GlcCer in normal mice, we compared 17 with Sanofi clinical candidate venglustat (Genz-682452) in the CBE mouse model of Gaucher disease type 3. At doses of 10 mg/kg, 17 and venglustat effected comparable reductions in both brain GlcCer and glucosylsphingosine. Importantly, 17 achieved these equivalent pharmacodynamic effects at significantly lower brain exposure than venglustat.
Keywords: Gaucher disease; Glucosylceramide synthase; blood−brain barrier; eliglustat tartrate.
Figures
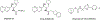
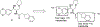
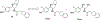
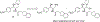

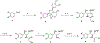
Similar articles
-
Venglustat combined with imiglucerase for neurological disease in adults with Gaucher disease type 3: the LEAP trial.Brain. 2023 Feb 13;146(2):461-474. doi: 10.1093/brain/awac379. Brain. 2023. PMID: 36256599 Free PMC article.
-
Eliglustat tartrate for the treatment of adults with type 1 Gaucher disease.Drug Des Devel Ther. 2015 Aug 18;9:4639-47. doi: 10.2147/DDDT.S77760. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26345314 Free PMC article. Review.
-
Recommendations for the use of eliglustat in the treatment of adults with Gaucher disease type 1 in the United States.Mol Genet Metab. 2016 Feb;117(2):95-103. doi: 10.1016/j.ymgme.2015.09.002. Epub 2015 Sep 7. Mol Genet Metab. 2016. PMID: 26387627 Review.
-
Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases.Curr Opin Investig Drugs. 2010 Oct;11(10):1169-81. Curr Opin Investig Drugs. 2010. PMID: 20872320 Review.
-
Inhibition of UDP-glucosylceramide synthase in mice prevents Gaucher disease-associated B-cell malignancy.J Pathol. 2015 Jan;235(1):113-24. doi: 10.1002/path.4452. J Pathol. 2015. PMID: 25256118
Cited by
-
Simultaneous Inhibition of Ceramide Hydrolysis and Glycosylation Synergizes to Corrupt Mitochondrial Respiration and Signal Caspase Driven Cell Death in Drug-Resistant Acute Myeloid Leukemia.Cancers (Basel). 2023 Mar 21;15(6):1883. doi: 10.3390/cancers15061883. Cancers (Basel). 2023. PMID: 36980769 Free PMC article.
-
Systematic Review of Genetic Substrate Reduction Therapy in Lysosomal Storage Diseases: Opportunities, Challenges and Delivery Systems.BioDrugs. 2024 Sep;38(5):657-680. doi: 10.1007/s40259-024-00674-1. Epub 2024 Aug 23. BioDrugs. 2024. PMID: 39177875 Free PMC article.
-
Diagnosis and Treatment for Shiga Toxin-Producing Escherichia coli Associated Hemolytic Uremic Syndrome.Toxins (Basel). 2022 Dec 23;15(1):10. doi: 10.3390/toxins15010010. Toxins (Basel). 2022. PMID: 36668830 Free PMC article. Review.
-
Neuroinflammation in neuronopathic Gaucher disease: Role of microglia and NK cells, biomarkers, and response to substrate reduction therapy.Elife. 2022 Aug 16;11:e79830. doi: 10.7554/eLife.79830. Elife. 2022. PMID: 35972072 Free PMC article.
-
Advancements in Viral Gene Therapy for Gaucher Disease.Genes (Basel). 2024 Mar 15;15(3):364. doi: 10.3390/genes15030364. Genes (Basel). 2024. PMID: 38540423 Free PMC article. Review.
References
-
- Platt FM, d’Azzo A, Davidson BL, Neufeld EF, and Tifft CJ (2018) Lysosomal storage diseases. Nat. Rev. Dis. Primers 4 (1), 27. - PubMed
-
- Shayman JA (2013) Eliglustat tartrate, a prototypic glucosylceramide synthase inhibitor. Expert Rev. Endocrinol. Metab. 8 (6), 491–504. - PubMed
-
- Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, Lukina E, Rosenbloom B, Ross L, Angell J, and Puga AC (2015) Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet 385 (9985), 2355–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

