Circulating Biomarkers of Accelerated Sarcopenia in Respiratory Diseases
- PMID: 33023021
- PMCID: PMC7600620
- DOI: 10.3390/biology9100322
Circulating Biomarkers of Accelerated Sarcopenia in Respiratory Diseases
Abstract
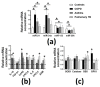
Skeletal muscle dysfunction is a critical finding in many respiratory diseases. However, a definitive biomarker to assess muscle decline in respiratory diseases is not known. We analyzed the association of plasma levels of glycoprotein Dickkopf-3 (Dkk-3), c-terminal agrin fragment-22 (CAF22) and microRNAs miR-21, miR-134a, miR-133 and miR-206 with hand-grip strength (HGS) and appendicular skeletal mass index (ASMI) in male, 54-73-year-old patients with chronic obstructive pulmonary diseases (COPD), asthma or pulmonary TB (n = 83-101/group). Patients with respiratory diseases showed a reduction in HGS and gait speed, while a reduction in ASMI was only found in patients with pulmonary TB. Among the sarcopenia indexes, HGS showed the strongest correlation with plasma CAF22, miR-21 and miR-206 levels while ASMI showed the strongest correlation with Dkk-3 and miR-133 in respiratory diseases. We found a modest-to-significant increase in the plasma markers of inflammation, oxidative stress and muscle damage, which had varying degrees of correlations with Dkk-3, CAF22 and selected micro RNAs (miRs) in respiratory diseases. Taken together, our data show that plasma levels of Dkk-3, CAF22 and selected miRs can be useful tools to assess accelerated sarcopenia phenotype in the elderly with respiratory diseases.
Keywords: CAF22; COPD; Dkk-3; asthma; miRs; sarcopenia; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


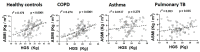
Similar articles
-
Plasma CAF22 Levels as a Useful Predictor of Muscle Health in Patients with Chronic Obstructive Pulmonary Disease.Biology (Basel). 2020 Jul 15;9(7):166. doi: 10.3390/biology9070166. Biology (Basel). 2020. PMID: 32679792 Free PMC article.
-
Circulating MicroRNAs as Biomarkers of Accelerated Sarcopenia in Chronic Heart Failure.Glob Heart. 2021 Aug 30;16(1):56. doi: 10.5334/gh.943. eCollection 2021. Glob Heart. 2021. PMID: 34692380 Free PMC article.
-
Elevated plasma zonulin and CAF22 are correlated with sarcopenia and functional dependency at various stages of Alzheimer's diseases.Neurosci Res. 2022 Nov;184:47-53. doi: 10.1016/j.neures.2022.08.004. Epub 2022 Aug 5. Neurosci Res. 2022. PMID: 35940439
-
Sarcopenia and frailty in chronic respiratory disease.Chron Respir Dis. 2017 Feb;14(1):85-99. doi: 10.1177/1479972316679664. Epub 2017 Feb 24. Chron Respir Dis. 2017. PMID: 27923981 Free PMC article. Review.
-
Handgrip Strength and Pulmonary Disease in the Elderly: What is the Link?Aging Dis. 2019 Oct 1;10(5):1109-1129. doi: 10.14336/AD.2018.1226. eCollection 2019 Oct. Aging Dis. 2019. PMID: 31595206 Free PMC article. Review.
Cited by
-
The Double-Edged Sword of ROS in Muscle Wasting and COPD: Insights from Aging-Related Sarcopenia.Antioxidants (Basel). 2024 Jul 22;13(7):882. doi: 10.3390/antiox13070882. Antioxidants (Basel). 2024. PMID: 39061950 Free PMC article.
-
Serum transthyretin and aminotransferases are associated with lean mass in people with coronary heart disease: Further insights from the CARE-CR study.Front Med (Lausanne). 2023 Feb 20;10:1094733. doi: 10.3389/fmed.2023.1094733. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36891188 Free PMC article.
-
Growth Differentiation Factor-15 as a Biomarker for Sarcopenia in Patients With Chronic Obstructive Pulmonary Disease.Front Nutr. 2022 Jun 30;9:897097. doi: 10.3389/fnut.2022.897097. eCollection 2022. Front Nutr. 2022. PMID: 35845807 Free PMC article.
-
Molecular Regulation of Skeletal Muscle Growth and Organelle Biosynthesis: Practical Recommendations for Exercise Training.Int J Mol Sci. 2021 Mar 8;22(5):2741. doi: 10.3390/ijms22052741. Int J Mol Sci. 2021. PMID: 33800501 Free PMC article. Review.
-
C-terminal agrin fragment as a biomarker of muscle wasting and weakness: a narrative review.J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):730-744. doi: 10.1002/jcsm.13189. Epub 2023 Feb 11. J Cachexia Sarcopenia Muscle. 2023. PMID: 36772862 Free PMC article. Review.
References
-
- Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. - DOI - PMC - PubMed
-
- Fujimoto K., Inage K., Eguchi Y., Orita S., Suzuki M., Kubota G., Sainoh T., Sato J., Shiga Y., Abe K., et al. Use of Bioelectrical Impedance Analysis for the Measurement of Appendicular Skeletal Muscle Mass/Whole Fat Mass and Its Relevance in Assessing Osteoporosis among Patients with Low Back Pain: A Comparative Analysis Using Dual X-ray Absorptiometry. Asian Spine J. 2018;12:839–845. doi: 10.31616/asj.2018.12.5.839. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

