Host pre-conditioning improves human adipose-derived stem cell transplantation in ageing rats after myocardial infarction: Role of NLRP3 inflammasome
- PMID: 33022900
- PMCID: PMC7686984
- DOI: 10.1111/jcmm.15403
Host pre-conditioning improves human adipose-derived stem cell transplantation in ageing rats after myocardial infarction: Role of NLRP3 inflammasome
Abstract
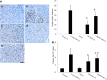
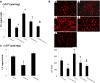
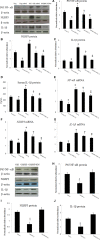
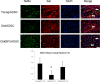
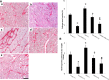
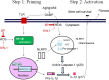
Functional decline of stem cell transplantation in ageing hosts is well documented. The mechanism for this is poorly understood, although it is known that advancing age does not provide an optimal milieu for exogenous stem cells to survive, engraft and differentiate. We showed that n-butylidenephthalide improved human adipose-derived stem cell (hADSC) engraftment via attenuating the production of reactive oxygen species (ROS). It remained unclear whether pre-treated hosts with n-butylidenephthalide can rejuvenate the ageing heart and improve hADSC engraftment by regulating the ROS/NLRP3 inflammasome-mediated cardiac fibrosis after myocardial infarction. One hour after coronary ligation, hADSCs were transplanted into the hearts of young and ageing Wistar rats that were pre-treated with or without n-butylidenephthalide for 3 days. At day 3 after infarction, myocardial infarction was associated with an increase in ROS levels and NLRP3 inflammasome activity with age. hADSC transplant effectively provided a significant decrease in ROS levels, NLRP3 inflammasome activity, IL-1β levels and cardiac fibrosis in either young or old infarcted rats. However, the beneficial effects of hADSCs were greater in young compared with old rats in terms of NLRP3 inflammasome activity. The infarcted ageing rats pre-conditioned by n-butylidenephthalide improved engraftment and differentiation of hADSCs and additionally attenuated cardiac fibrosis compared with hADSCs alone. The anti-inflammation effects of n-butylidenephthalide were reversed by SIN-1. In conclusions, the increased NLRP3 inflammasome activity plays the pathogenesis of ageing-related functional hADSC decline in the ageing hosts. n-butylidenephthalide-pre-treated ageing hosts reversibly ameliorate the harsh microenvironments, improve stem cell engraftment and attenuate cardiac fibrosis after myocardial infarction.
Keywords: NLRP3 inflammasome; adipose-derived stem cell; butylidenephthalide; myocardial fibrosis; reactive oxygen species.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

Similar articles
-
Intramyocardial injection of human adipose-derived stem cells ameliorates cognitive deficit by regulating oxidative stress-mediated hippocampal damage after myocardial infarction.J Mol Med (Berl). 2021 Dec;99(12):1815-1827. doi: 10.1007/s00109-021-02135-6. Epub 2021 Oct 11. J Mol Med (Berl). 2021. PMID: 34633469 Free PMC article.
-
Remote transplantation of human adipose-derived stem cells induces regression of cardiac hypertrophy by regulating the macrophage polarization in spontaneously hypertensive rats.Redox Biol. 2019 Oct;27:101170. doi: 10.1016/j.redox.2019.101170. Epub 2019 Mar 21. Redox Biol. 2019. PMID: 31164286 Free PMC article.
-
Targeting the pathway of GSK-3β/nerve growth factor to attenuate post-infarction arrhythmias by preconditioned adipose-derived stem cells.J Mol Cell Cardiol. 2017 Mar;104:17-30. doi: 10.1016/j.yjmcc.2017.01.014. Epub 2017 Jan 24. J Mol Cell Cardiol. 2017. PMID: 28130118
-
Role of NLRP3 Inflammasome in Cardiac Inflammation and Remodeling after Myocardial Infarction.Biol Pharm Bull. 2019;42(4):518-523. doi: 10.1248/bpb.b18-00369. Biol Pharm Bull. 2019. PMID: 30930410 Review.
-
Regulation and functions of NLRP3 inflammasome in cardiac fibrosis: Current knowledge and clinical significance.Biomed Pharmacother. 2021 Nov;143:112219. doi: 10.1016/j.biopha.2021.112219. Epub 2021 Sep 21. Biomed Pharmacother. 2021. PMID: 34560540 Review.
Cited by
-
Potential Therapies to Protect the Aging Heart Against Ischemia/Reperfusion Injury.Front Cardiovasc Med. 2021 Nov 19;8:770421. doi: 10.3389/fcvm.2021.770421. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34869687 Free PMC article. Review.
-
Signaling pathways and targeted therapy for myocardial infarction.Signal Transduct Target Ther. 2022 Mar 10;7(1):78. doi: 10.1038/s41392-022-00925-z. Signal Transduct Target Ther. 2022. PMID: 35273164 Free PMC article. Review.
-
Application of adipose-derived stem cells in treating fibrosis.World J Stem Cells. 2021 Nov 26;13(11):1747-1761. doi: 10.4252/wjsc.v13.i11.1747. World J Stem Cells. 2021. PMID: 34909121 Free PMC article. Review.
-
Post-myocardial infarction fibrosis: Pathophysiology, examination, and intervention.Front Pharmacol. 2023 Mar 28;14:1070973. doi: 10.3389/fphar.2023.1070973. eCollection 2023. Front Pharmacol. 2023. PMID: 37056987 Free PMC article. Review.
-
The Protective Effects of n-Butylidenephthalide on Retinal Ganglion Cells during Ischemic Injury.Int J Mol Sci. 2022 Feb 14;23(4):2095. doi: 10.3390/ijms23042095. Int J Mol Sci. 2022. PMID: 35216208 Free PMC article.
References
-
- Gould KE, Taffet GE, Michael LH, et al. Heart failure and greater infarct expansion in middle aged mice: a relevant model for postinfarction failure. Am J Physiol Heart Circ Physiol. 2002;282:H615‐H621. - PubMed
-
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256(6 Pt 1):C1262‐C1266. - PubMed
-
- Smythe GM, Shavlakadze T, Roberts P, et al. Age influences the early events of skeletal muscle regeneration: studies of whole muscle grafts transplanted between young (8 weeks) and old (13–21 months) mice. Exp Gerontol. 2008;43:550‐562. - PubMed
-
- Mauro AG, Bonaventura A, Mezzaroma E, Quader M, Toldo S. NLRP3 inflammasome in acute myocardial infarction. J Cardiovasc Pharmacol. 2019;74:175‐187. - PubMed
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821‐832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

