Electrostatic Surface Properties of Blood and Semen Extracellular Vesicles: Implications of Sialylation and HIV-Induced Changes on EV Internalization
- PMID: 33019624
- PMCID: PMC7601085
- DOI: 10.3390/v12101117
Electrostatic Surface Properties of Blood and Semen Extracellular Vesicles: Implications of Sialylation and HIV-Induced Changes on EV Internalization
Abstract
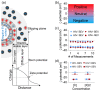
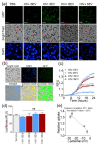
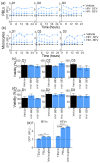
Although extracellular vesicle (EV) surface electrostatic properties (measured as zeta potential, ζ-potential) have been reported by many investigators, the biophysical implications of charge and EV origin remains uncertain. Here, we compared the ζ-potential of human blood EVs (BEVs) and semen EVs (SEVs) from 26 donors that were HIV-infected (HIV+, n = 13) or HIV uninfected (HIV-, n = 13). We found that, compared to BEVs that bear neutral surface charge, SEVs were significantly more negatively charged, even when BEVs and SEVs were from the same individual. Comparison of BEVs and SEVs from HIV- and HIV+ groups revealed subtle HIV-induced alteration in the ζ-potential of EVs, with the effect being more significant in SEVs (∆ζ-potential = -8.82 mV, p-value = 0.0062) than BEVs (∆ζ-potential = -1.4 mV, p-value = 0.0462). These observations were validated by differences in the isoelectric point (IEP) of EVs, which was in the order of HIV + SEV ≤ HIV-SEV ≪ HIV + BEV ≤ HIV-BEV. Functionally, the rate and efficiency of SEV internalization by the human cervical epithelial cell line, primary peripheral blood lymphocytes, and primary blood-derived monocytes were significantly higher than those of BEVs. Mechanistically, removal of sialic acids from the surface of EVs using neuraminidase treatment significantly decreased SEV's surface charge, concomitant with a substantial reduction in SEV's internalization. The neuraminidase effect was independent of HIV infection and insignificant for BEVs. Finally, these results were corroborated by enrichment of glycoproteins in SEVs versus BEVs. Taken together, these findings uncover fundamental tissue-specific differences in surface electrostatic properties of EVs and highlight the critical role of surface charge in EV/target cell interactions.
Keywords: HIV-1; biological membranes; blood; extracellular vesicles; glycocalyx; semen; zeta potential.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Membrane Protein Modification Modulates Big and Small Extracellular Vesicle Biodistribution and Tumorigenic Potential in Breast Cancers In Vivo.Adv Mater. 2023 Mar;35(13):e2208966. doi: 10.1002/adma.202208966. Epub 2023 Feb 13. Adv Mater. 2023. PMID: 36609913
-
Seminal Plasma-Derived Extracellular-Vesicle Fractions from HIV-Infected Men Exhibit Unique MicroRNA Signatures and Induce a Proinflammatory Response in Cells Isolated from the Female Reproductive Tract.J Virol. 2020 Jul 30;94(16):e00525-20. doi: 10.1128/JVI.00525-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32434889 Free PMC article.
-
Long-Term Low-Dose Delta-9-Tetrahydrocannbinol (THC) Administration to Simian Immunodeficiency Virus (SIV) Infected Rhesus Macaques Stimulates the Release of Bioactive Blood Extracellular Vesicles (EVs) that Induce Divergent Structural Adaptations and Signaling Cues.Cells. 2020 Oct 6;9(10):2243. doi: 10.3390/cells9102243. Cells. 2020. PMID: 33036231 Free PMC article.
-
Unveiling clinical applications of bacterial extracellular vesicles as natural nanomaterials in disease diagnosis and therapeutics.Acta Biomater. 2024 May;180:18-45. doi: 10.1016/j.actbio.2024.04.022. Epub 2024 Apr 17. Acta Biomater. 2024. PMID: 38641182 Review.
-
Proteomics analysis of circulating small extracellular vesicles: Focus on the contribution of EVs to tumor metabolism.Cytokine Growth Factor Rev. 2023 Oct;73:3-19. doi: 10.1016/j.cytogfr.2023.08.003. Epub 2023 Aug 19. Cytokine Growth Factor Rev. 2023. PMID: 37652834 Review.
Cited by
-
Do extracellular vesicles have specific target cells?; Extracellular vesicle mediated embryo maternal communication.Front Mol Biosci. 2024 Jul 16;11:1415909. doi: 10.3389/fmolb.2024.1415909. eCollection 2024. Front Mol Biosci. 2024. PMID: 39081929 Free PMC article.
-
Blood plasma derived extracellular vesicles (BEVs): particle purification liquid chromatography (PPLC) and proteomic analysis reveals BEVs as a potential minimally invasive tool for predicting response to breast cancer treatment.Breast Cancer Res Treat. 2022 Nov;196(2):423-437. doi: 10.1007/s10549-022-06733-x. Epub 2022 Sep 17. Breast Cancer Res Treat. 2022. PMID: 36114323 Free PMC article.
-
Application of engineered extracellular vesicles for targeted tumor therapy.J Biomed Sci. 2022 Feb 21;29(1):14. doi: 10.1186/s12929-022-00798-y. J Biomed Sci. 2022. PMID: 35189894 Free PMC article. Review.
-
Protective role of engineered extracellular vesicles loaded quercetin nanoparticles as anti-viral therapy against SARS-CoV-2 infection: A prospective review.Front Immunol. 2022 Dec 8;13:1040027. doi: 10.3389/fimmu.2022.1040027. eCollection 2022. Front Immunol. 2022. PMID: 36569877 Free PMC article. Review.
-
Neural stem cell-derived exosomes and regeneration: cell-free therapeutic strategies for traumatic brain injury.Stem Cell Res Ther. 2023 Aug 8;14(1):198. doi: 10.1186/s13287-023-03409-1. Stem Cell Res Ther. 2023. PMID: 37553595 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

