Intravital longitudinal imaging of hepatic lipid droplet accumulation in a murine model for nonalcoholic fatty liver disease
- PMID: 33014604
- PMCID: PMC7510864
- DOI: 10.1364/BOE.395890
Intravital longitudinal imaging of hepatic lipid droplet accumulation in a murine model for nonalcoholic fatty liver disease
Abstract
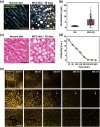
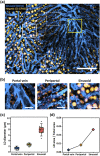
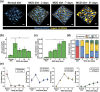
Nonalcoholic fatty liver disease (NAFLD) is a rapidly increasing chronic liver disorder worldwide accompanied by hepatic steatosis, inflammation, fibrosis, and severe liver failure. Unfortunately, an effective treatment strategy for NAFLD has not yet been established, which has been hampered by the limited understanding of the pathophysiological drivers for NAFLD. To examine the unknown cellular and molecular mechanisms in the pathogenesis of NAFLD, there is an increasing need for the direct in vivo observation of hepatic microenvironments over extended periods of time. In this work, using a custom-built intravital imaging system and a novel fluorescent lipid droplet labeling dye, Seoul-Fluor 44 (SF44), we established an intravital imaging method to visualize individual lipid droplets and microvasculature simultaneously in the liver of live mice in vivo. In addition, in the nonalcoholic steatosis and steatohepatitis mouse model induced by a methionine and choline-deficient diet, we longitudinally visualized and quantitatively analyzed the development of lipid droplets in hepatocytes and sinusoid at a subcellular resolution during the progression of NAFLD up to 21 days in vivo.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures



Similar articles
-
Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State.J Lipid Atheroscler. 2021 Sep;10(3):313-321. doi: 10.12997/jla.2021.10.3.313. Epub 2021 Jun 28. J Lipid Atheroscler. 2021. PMID: 34621702 Free PMC article.
-
Intravital two-photon imaging and quantification of hepatic steatosis and fibrosis in a live small animal model.Biomed Opt Express. 2021 Dec 1;12(12):7918-7927. doi: 10.1364/BOE.442608. eCollection 2021 Dec 1. Biomed Opt Express. 2021. PMID: 35003876 Free PMC article.
-
Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis.Hepatology. 2007 Jun;45(6):1366-74. doi: 10.1002/hep.21655. Hepatology. 2007. PMID: 17476695
-
Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease.Annu Rev Pathol. 2018 Jan 24;13:321-350. doi: 10.1146/annurev-pathol-020117-043617. Annu Rev Pathol. 2018. PMID: 29414249 Review.
-
Fatty Acids and Effects on In Vitro and In Vivo Models of Liver Steatosis.Curr Med Chem. 2019;26(19):3439-3456. doi: 10.2174/0929867324666170518101334. Curr Med Chem. 2019. PMID: 28521680 Review.
Cited by
-
Intravital Imaging of Inflammatory Response in Liver Disease.Front Cell Dev Biol. 2022 Jun 28;10:922041. doi: 10.3389/fcell.2022.922041. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35837329 Free PMC article. Review.
-
Intravital Two-photon Imaging of Dynamic Alteration of Hepatic Lipid Droplets in Fasted and Refed State.J Lipid Atheroscler. 2021 Sep;10(3):313-321. doi: 10.12997/jla.2021.10.3.313. Epub 2021 Jun 28. J Lipid Atheroscler. 2021. PMID: 34621702 Free PMC article.
-
Three-dimensional imaging and analysis of pathological tissue samples with de novo generation of citrate-based fluorophores.Sci Adv. 2022 Nov 18;8(46):eadd9419. doi: 10.1126/sciadv.add9419. Epub 2022 Nov 16. Sci Adv. 2022. PMID: 36383671 Free PMC article.
-
Evaluation Through the Optical Coherence Tomography Analysis of the Influence of Non-Alcoholic Fatty Liver Disease on the Gingival Inflammation in Periodontal Patients.Diabetes Metab Syndr Obes. 2021 Jun 29;14:2935-2942. doi: 10.2147/DMSO.S310314. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34234491 Free PMC article.
-
In vivo longitudinal 920 nm two-photon intravital kidney imaging of a dynamic 2,8-DHA crystal formation and tubular deterioration in the adenine-induced chronic kidney disease mouse model.Biomed Opt Express. 2023 Mar 27;14(4):1647-1658. doi: 10.1364/BOE.485187. eCollection 2023 Apr 1. Biomed Opt Express. 2023. PMID: 37078028 Free PMC article.
References
LinkOut - more resources
Full Text Sources
