Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis
- PMID: 33008073
- PMCID: PMC7600854
- DOI: 10.3390/cells9102218
Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis
Abstract
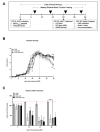
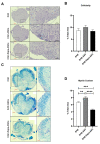
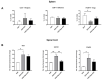
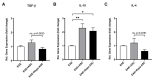
Human adipose-derived stem cells (ASCs) show immense promise for treating inflammatory diseases, attributed primarily to their potent paracrine signaling. Previous investigations demonstrated that short-term Rapamycin preconditioning of bone marrow-derived stem cells (BMSCs) elevated secretion of prostaglandin E2, a pleiotropic molecule with therapeutic effects in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS), and enhanced immunosuppressive capacity in vitro. However, this has yet to be examined in ASCs. The present study examined the therapeutic potential of short-term Rapamycin-preconditioned ASCs in the EAE model. Animals were treated at peak disease with control ASCs (EAE-ASCs), Rapa-preconditioned ASCs (EAE-Rapa-ASCs), or vehicle control (EAE). Results show that EAE-ASCs improved clinical disease scores and elevated intact myelin compared to both EAE and EAE-Rapa-ASC animals. These results correlated with augmented CD4+ T helper (Th) and T regulatory (Treg) cell populations in the spinal cord, and increased gene expression of interleukin-10 (IL-10), an anti-inflammatory cytokine. Conversely, EAE-Rapa-ASC mice showed no improvement in clinical disease scores, reduced myelin levels, and significantly less Th and Treg cells in the spinal cord. These findings suggest that short-term Rapamycin preconditioning reduces the therapeutic efficacy of ASCs when applied to late-stage EAE.
Keywords: Rapamycin; adipose tissue-derived stem cells (ASCs); demyelination; experimental autoimmune encephalomyelitis (EAE); immunomodulation; inflammation; multiple sclerosis (MS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model.IUBMB Life. 2016 Feb;68(2):106-15. doi: 10.1002/iub.1469. Epub 2016 Jan 12. IUBMB Life. 2016. PMID: 26757144 Review.
-
Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis.Stem Cells. 2009 Oct;27(10):2624-35. doi: 10.1002/stem.194. Stem Cells. 2009. PMID: 19676124
-
Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model.Stem Cells Transl Med. 2013 Oct;2(10):797-807. doi: 10.5966/sctm.2013-0026. Epub 2013 Sep 9. Stem Cells Transl Med. 2013. PMID: 24018793 Free PMC article.
-
Transplantation of autologous adipose stem cells lacks therapeutic efficacy in the experimental autoimmune encephalomyelitis model.PLoS One. 2014 Jan 21;9(1):e85007. doi: 10.1371/journal.pone.0085007. eCollection 2014. PLoS One. 2014. PMID: 24465465 Free PMC article.
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
Short-Term Autophagy Preconditioning Upregulates the Expression of COX2 and PGE2 and Alters the Immune Phenotype of Human Adipose-Derived Stem Cells In Vitro.Cells. 2022 Apr 19;11(9):1376. doi: 10.3390/cells11091376. Cells. 2022. PMID: 35563682 Free PMC article.
-
Adipose-Derived Stem Cell Exosomes Inhibit Hypertrophic Scaring Formation by Regulating Th17/Treg Cell Balance.Biomed Res Int. 2022 Oct 12;2022:9899135. doi: 10.1155/2022/9899135. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Jul 12;2023:9897257. doi: 10.1155/2023/9897257. PMID: 36277890 Free PMC article. Retracted.
-
Secretome as a Tool to Treat Neurological Conditions: Are We Ready?Int J Mol Sci. 2023 Nov 20;24(22):16544. doi: 10.3390/ijms242216544. Int J Mol Sci. 2023. PMID: 38003733 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

