Heteromerization of Endogenous Mu and Delta Opioid Receptors Induces Ligand-Selective Co-Targeting to Lysosomes
- PMID: 33007971
- PMCID: PMC7583997
- DOI: 10.3390/molecules25194493
Heteromerization of Endogenous Mu and Delta Opioid Receptors Induces Ligand-Selective Co-Targeting to Lysosomes
Abstract
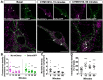
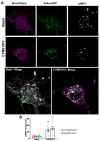
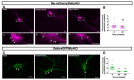
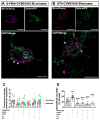
Increasing evidence indicates that native mu and delta opioid receptors can associate to form heteromers in discrete brain neuronal circuits. However, little is known about their signaling and trafficking. Using double-fluorescent knock-in mice, we investigated the impact of neuronal co-expression on the internalization profile of mu and delta opioid receptors in primary hippocampal cultures. We established ligand selective mu-delta co-internalization upon activation by 1-[[4-(acetylamino)phenyl]methyl]-4-(2-phenylethyl)-4-piperidinecarboxylic acid, ethyl ester (CYM51010), [d-Ala2, NMe-Phe4, Gly-ol5]enkephalin (DAMGO), and deltorphin II, but not (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80), morphine, or methadone. Co-internalization was driven by the delta opioid receptor, required an active conformation of both receptors, and led to sorting to the lysosomal compartment. Altogether, our data indicate that mu-delta co-expression, likely through heteromerization, alters the intracellular fate of the mu opioid receptor, which provides a way to fine-tune mu opioid receptor signaling. It also represents an interesting emerging concept for the development of novel therapeutic drugs and strategies.
Keywords: delta opioid receptor; heteromer; internalization; lysosomes; mu opioid receptor; primary hippocampal culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking.J Pharmacol Exp Ther. 2011 Jun;337(3):868-75. doi: 10.1124/jpet.111.179093. Epub 2011 Mar 21. J Pharmacol Exp Ther. 2011. PMID: 21422164 Free PMC article.
-
Prolonged morphine treatment alters δ opioid receptor post-internalization trafficking.Br J Pharmacol. 2015 Jan;172(2):615-29. doi: 10.1111/bph.12761. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24819092 Free PMC article.
-
Diaryldimethylpiperazine ligands with mu- and delta-opioid receptor affinity: Synthesis of (+)-4-[(alphaR)-alpha-(4-allyl-(2S,5S)-dimethylpiperazin-1-yl)-(3-hydroxyphenyl)methyl]-N-ethyl-N-phenylbenzamide and (-)-4-[(alphaR)-alpha-(2S,5S)-dimethylpiperazin-1-yl)-(3-hydroxyphenyl)methyl]-N-ethyl-N-phenylbenzamide.Bioorg Med Chem. 2003 Nov 3;11(22):4761-8. doi: 10.1016/s0968-0896(03)00496-6. Bioorg Med Chem. 2003. PMID: 14556791
-
Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities.Br J Pharmacol. 2015 Jan;172(2):375-87. doi: 10.1111/bph.12663. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24571499 Free PMC article. Review.
-
Functional relevance of μ-δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology.Drug Alcohol Depend. 2012 Mar 1;121(3):167-72. doi: 10.1016/j.drugalcdep.2011.10.025. Epub 2011 Nov 23. Drug Alcohol Depend. 2012. PMID: 22115888 Free PMC article. Review.
Cited by
-
Mu opioid receptor-mediated release of endolysosome iron increases levels of mitochondrial iron, reactive oxygen species, and cell death.NeuroImmune Pharm Ther. 2023 Mar 25;2(1):19-35. doi: 10.1515/nipt-2022-0013. Epub 2022 Sep 14. NeuroImmune Pharm Ther. 2023. PMID: 37027339 Free PMC article.
-
Opioids and Their Receptors: Present and Emerging Concepts in Opioid Drug Discovery.Molecules. 2020 Dec 1;25(23):5658. doi: 10.3390/molecules25235658. Molecules. 2020. PMID: 33271753 Free PMC article.
-
Knock-In Mouse Models to Investigate the Functions of Opioid Receptors in vivo.Front Cell Neurosci. 2022 Jan 31;16:807549. doi: 10.3389/fncel.2022.807549. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35173584 Free PMC article. Review.
-
Endogenous opioid systems alterations in pain and opioid use disorder.Front Syst Neurosci. 2022 Oct 19;16:1014768. doi: 10.3389/fnsys.2022.1014768. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36341476 Free PMC article. Review.
-
Activation of the Mu-Delta Opioid Receptor Heteromers Blocks Morphine Rewarding Effects.Int J Neuropsychopharmacol. 2023 Jul 31;26(7):513-521. doi: 10.1093/ijnp/pyad032. Int J Neuropsychopharmacol. 2023. PMID: 37343217 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

