Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?
- PMID: 33007839
- PMCID: PMC7712059
- DOI: 10.3390/jof6040198
Modeling Invasive Aspergillosis: How Close Are Predicted Antifungal Targets?
Abstract
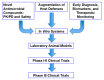
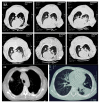
Animal model systems are a critical component of the process of discovery and development of new antifungal agents for treatment and prevention of invasive aspergillosis. The persistently neutropenic rabbit model of invasive pulmonary aspergillosis (IPA) has been a highly predictive system in identifying new antifungal agents for treatment and prevention of this frequently lethal infection. Since its initial development, the persistently neutropenic rabbit model of IPA has established a strong preclinical foundation for dosages, drug disposition, pharmacokinetics, safety, tolerability, and efficacy for deoxycholate amphotericin B, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B colloidal dispersion, caspofungin, micafungin, anidulafungin, voriconazole, posaconazole, isavuconazole, and ibrexafungerp in treatment of patients with invasive aspergillosis. The findings of combination therapy with a mould-active triazole and an echinocandin in this rabbit model also predicted the outcome of the clinical trial for voriconazole plus anidulafungin for treatment of IPA. The plasma pharmacokinetic parameters and tissue disposition for most antifungal agents approximate those of humans in persistently neutropenic rabbits. Safety, particularly nephrotoxicity, has also been highly predictive in the rabbit model, as exemplified by the differential glomerular filtration rates observed in animals treated with deoxycholate amphotericin B, liposomal amphotericin B, amphotericin B lipid complex, and amphotericin B colloidal dispersion. A panel of validated outcome variables measures therapeutic outcome in the rabbit model: residual fungal burden, markers of organism-mediated pulmonary injury (lung weights and infarct scores), survival, and serum biomarkers. In selected antifungal studies, thoracic computerized tomography (CT) is also used with diagnostic imaging algorithms to measure therapeutic response of pulmonary infiltrates, which exhibit characteristic radiographic patterns, including nodules and halo signs. Further strengthening the predictive properties of the model, therapeutic response to successfully developed antifungal agents for treatment of IPA has been demonstrated over the past two decades by biomarkers of serum galactomannan and (1→3)-β-D-glucan with patterns of resolution, that closely mirror those documented responses in patients with IPA. The decision to move from laboratory to clinical trials should be predicated upon a portfolio of complementary and mutually validating preclinical laboratory animal models studies. Other model systems, including those in mice, rats, and guinea pigs, are also valuable tools in developing clinical protocols. Meticulous preclinical investigation of a candidate antifungal compound in a robust series of complementary laboratory animal models will optimize study design, de-risk clinical trials, and ensure tangible benefit to our most vulnerable immunocompromised patients with invasive aspergillosis.
Keywords: animal models; antifungal agents; aspergillosis; translational medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
[Expert consensus on the diagnosis and treatment of pulmonary aspergillosis in patients with chronic obstructive pulmonary disease].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Jul 12;47(7):604-622. doi: 10.3760/cma.j.cn112147-20231228-00399. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38955746 Chinese.
-
Combination Therapy with Ibrexafungerp (Formerly SCY-078), a First-in-Class Triterpenoid Inhibitor of (1→3)-β-d-Glucan Synthesis, and Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis.Antimicrob Agents Chemother. 2020 May 21;64(6):e02429-19. doi: 10.1128/AAC.02429-19. Print 2020 May 21. Antimicrob Agents Chemother. 2020. PMID: 32179521 Free PMC article.
-
Combination Therapy with Isavuconazole and Micafungin for Treatment of Experimental Invasive Pulmonary Aspergillosis.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00305-17. doi: 10.1128/AAC.00305-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28696236 Free PMC article.
-
Invasive Pulmonary Aspergillosis.Semin Respir Crit Care Med. 2020 Feb;41(1):80-98. doi: 10.1055/s-0039-3401990. Epub 2020 Jan 30. Semin Respir Crit Care Med. 2020. PMID: 32000286 Review.
-
Caspofungin: pharmacology, safety and therapeutic potential in superficial and invasive fungal infections.Expert Opin Investig Drugs. 2001 Aug;10(8):1545-58. doi: 10.1517/13543784.10.8.1545. Expert Opin Investig Drugs. 2001. PMID: 11772269 Review.
Cited by
-
Dysregulation of Key Proteinases in Aspergillus fumigatus Induced by Blood Platelets.Rep Biochem Mol Biol. 2021 Apr;10(1):95-104. doi: 10.52547/rbmb.10.1.95. Rep Biochem Mol Biol. 2021. PMID: 34277873 Free PMC article.
References
-
- Maertens J.A., Raad I.I., Marr K.A., Patterson T.F., Kontoyiannis D.P., Cornely O.A., Bow E.J., Rahav G., Neofytos D., Aoun M., et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomized-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. - DOI - PubMed
-
- Lepak A.J., Marchillo K., Vanhecker J., Andes D.R. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2013;57:579–585. doi: 10.1128/AAC.01279-12. - DOI - PMC - PubMed
-
- Wiederhold N.P., Najvar L.K., Vallor A.C., Kirkpatrick W.R., Bocanegra R., Molina D., Olivo M., Graybill J.R., Patterson T.F. Assessment of serum (1->3)-β-D-glucan concentration as a measure of disease burden in a murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2008;52:1176–1178. doi: 10.1128/AAC.01425-07. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

