The construction of a lymphoma cell-based, DC-targeted vaccine, and its application in lymphoma prevention and cure
- PMID: 33005832
- PMCID: PMC7511651
- DOI: 10.1016/j.bioactmat.2020.09.002
The construction of a lymphoma cell-based, DC-targeted vaccine, and its application in lymphoma prevention and cure
Abstract
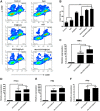
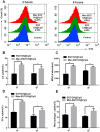
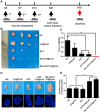
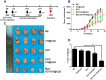
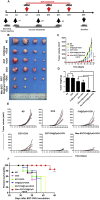
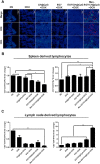
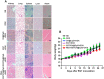
In recent years, Non-Hodgkin lymphoma (NHL) has been one of the most fast-growing malignant tumor diseases. NHL poses severe damages to physical health and a heavy burden to patients. Traditional therapies (chemotherapy or radiotherapy) bring some benefit to patients, but have severe adverse effects and do not prevent relapse. The relevance of emerging immunotherapy options (immune-checkpoint blockers or adoptive cellular methods) for NHL remains uncertain, and more intensive evaluations are needed. In this work, inspired by the idea of vaccination to promote an immune response to destroy tumors, we used a biomaterial-based strategy to improve a tumor cell-based vaccine and constructed a novel vaccine named Man-EG7/CH@CpG with antitumor properties. In this vaccine, natural tumor cells are used as a vector to load CpG-ODN, and following lethal irradiation, the formulations were decorated with mannose. The study of the characterization of the double-improved vaccine evidenced the enhanced ability of DCs targeting and improved immunocompetence, which displayed an antitumor function. In the lymphoma prevention model, the Man-EG7/CH@CpG vaccine restrained tumor formation with high efficiency. Furthermore, unlike the non-improved vaccine, the double-improved vaccine elicited an enhanced antitumor effect in the lymphoma treatment model. Next, to improve the moderate therapeutic effect of the mono-treatment method, we incorporated a chemotherapeutic drug (doxorubicin, DOX) into the process of vaccination and devised a combination regimen. Fortunately, a tumor inhibition rate of ~85% was achieved via the combination therapy, which could not be achieved by mono-chemotherapy or mono-immunotherapy. In summary, the strategy presented here may provide a novel direction in the establishment of a tumor vaccine and is the basis for a prioritization scheme of immuno-chemotherapy in enhancing the therapeutic effect on NHL.
Keywords: Combinational therapy; DCs targeting; Man-EG7/CH@CpG vaccine; Non-hodgkin lymphoma.
© 2020 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The enhanced antitumor-specific immune response with mannose- and CpG-ODN-coated liposomes delivering TRP2 peptide.Theranostics. 2018 Feb 12;8(6):1723-1739. doi: 10.7150/thno.22056. eCollection 2018. Theranostics. 2018. PMID: 29556352 Free PMC article.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
A Deep Insight Into CAR-T Cell Therapy in Non-Hodgkin Lymphoma: Application, Opportunities, and Future Directions.Front Immunol. 2021 Jun 23;12:681984. doi: 10.3389/fimmu.2021.681984. eCollection 2021. Front Immunol. 2021. Retraction in: Front Immunol. 2023 Sep 04;14:1285110. doi: 10.3389/fimmu.2023.1285110. PMID: 34248965 Free PMC article. Retracted. Review.
-
Mannose-Modified Liposome Co-Delivery of Human Papillomavirus Type 16 E7 Peptide and CpG Oligodeoxynucleotide Adjuvant Enhances Antitumor Activity Against Established Large TC-1 Grafted Tumors in Mice.Int J Nanomedicine. 2020 Dec 1;15:9571-9586. doi: 10.2147/IJN.S275670. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33293808 Free PMC article.
-
Allogeneic stem cell transplantation in aggressive B-cell non-Hodgkin lymphoma and in T-cell non-Hodgkin lymphoma: IQWiG Reports – Commission No. N17-02 [Internet].Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2019 Nov 19. Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2019 Nov 19. PMID: 31790167 Free Books & Documents. Review.
Cited by
-
Polysaccharide-based nanomedicines for cancer immunotherapy: A review.Bioact Mater. 2021 Mar 18;6(10):3358-3382. doi: 10.1016/j.bioactmat.2021.03.008. eCollection 2021 Oct. Bioact Mater. 2021. PMID: 33817416 Free PMC article. Review.
-
Modified ASO conjugates encapsulated with cytidinyl/cationic lipids exhibit more potent and longer-lasting anti-HCC effects.Mol Ther Nucleic Acids. 2023 May 4;32:807-821. doi: 10.1016/j.omtn.2023.04.028. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 37251692 Free PMC article.
-
A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers.Molecules. 2021 Jun 3;26(11):3382. doi: 10.3390/molecules26113382. Molecules. 2021. PMID: 34205019 Free PMC article. Review.
-
Advances in therapeutic cancer vaccines: Harnessing immune adjuvants for enhanced efficacy and future perspectives.Comput Struct Biotechnol J. 2024 Apr 21;23:1833-1843. doi: 10.1016/j.csbj.2024.04.054. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38707540 Free PMC article. Review.
References
-
- Armitage J.O., Gascoyne R.D., Lunning M.A., Cavalli F. 2017. Non-Hodgkin Lymphoma. - PubMed
-
- Shankland K.R., Armitage J.O., Hancock B.W. 2012. Non-Hodgkin Lymphoma. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020, CA. A Cancer Journal for Clinicians. 2020;70:7–30. - PubMed
-
- Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I., Abdulle A., Dereje N., Niguse H., Abu-Raddad L., Abualhasan A., Isaac A., Advani S., Afarideh M., Afshari M., Aghaali M., Ghajar A. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017 A systematic analysis for the global burden of disease study. JAMA Oncology. 2019;5:1749–1768. - PMC - PubMed
-
- Stillwell T.J., Benson R.C., Jr. Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988;61:451–457. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

