Chronic inflammatory pain alters alcohol-regulated frontocortical signaling and associations between alcohol drinking and thermal sensitivity
- PMID: 33005820
- PMCID: PMC7509777
- DOI: 10.1016/j.ynpai.2020.100052
Chronic inflammatory pain alters alcohol-regulated frontocortical signaling and associations between alcohol drinking and thermal sensitivity
Abstract
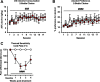
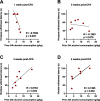
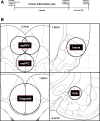
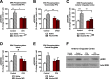
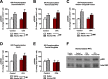
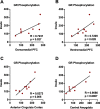
Alcohol use disorder (AUD) is a chronic, relapsing psychiatric disorder that is characterized by the emergence of negative affective states. The transition from recreational, limited intake to uncontrolled, escalated intake is proposed to involve a transition from positive to negative reinforcement mechanisms for seeking alcohol. Past work has identified the emergence of significant hyperalgesia/allodynia in alcohol-dependent animals, which may serve as a key negative reinforcement mechanism. Chronic pain has been associated with enhanced extracellular signal-regulated kinase (ERK) activity in cortical and subcortical nociceptive areas. Additionally, both pain and AUD have been associated with increased activity of the glucocorticoid receptor (GR), a key mediator of stress responsiveness. The objectives of the current study were to first determine relationships between thermal nociceptive sensitivity and alcohol drinking in male Wistar rats. While inflammatory pain induced by complete Freund's adjuvant (CFA) administration did not modify escalation of home cage drinking in animals over four weeks, the relationship between drinking levels and hyperalgesia symptoms reversed between acute (1 week) and chronic (3-4 week) periods post-CFA administration, suggesting that either the motivational or analgesic effects of alcohol may be altered over the time course of chronic pain. We next examined ERK and GR phosphorylation in pain-related brain areas (including the central amygdala and prefrontal cortex subregions) in animals experiencing acute withdrawal from binge alcohol administration (2 g/kg, 6 h withdrawal) and CFA administration (four weeks) to model the neurobiological consequences of binge alcohol exposure in the context of pain. We observed a significant interaction between alcohol and pain state, whereby alcohol withdrawal increased ERK phosphorylation across all four frontocortical areas examined, although this effect was absent in animals experiencing chronic inflammatory pain. Alcohol withdrawal also increased GR phosphorylation across all four frontocortical areas, but these changes were not altered by CFA. Interestingly, we observed significant inter-brain regional correlations in GR phosphorylation between the insula and other regions investigated only in animals exposed to both alcohol and CFA, suggesting coordinated activity in insula circuitry and glucocorticoid signaling in this context. The results of these studies provide a greater understanding of the neurobiology of AUD and will contribute to the development of effective treatment strategies for comorbid AUD and pain.
Keywords: Alcohol; Central amygdala; ERK; Glucocorticoid receptor; Pain; Prefrontal cortex.
© 2020 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Dysregulation of c-Jun N-terminal kinase phosphorylation in alcohol dependence.Alcohol. 2019 Mar;75:11-18. doi: 10.1016/j.alcohol.2018.04.006. Epub 2018 Apr 19. Alcohol. 2019. PMID: 30321699 Free PMC article.
-
Sex-specific biobehavioral regulation of persistent inflammatory pain by alcohol.Alcohol Clin Exp Res (Hoboken). 2023 Jul;47(7):1283-1296. doi: 10.1111/acer.15104. Epub 2023 May 30. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 37208939 Free PMC article.
-
Neurobiological aspects of pain in the context of alcohol use disorder.Int Rev Neurobiol. 2021;157:1-29. doi: 10.1016/bs.irn.2020.09.001. Epub 2020 Oct 6. Int Rev Neurobiol. 2021. PMID: 33648668 Free PMC article.
-
The Convergent Neuroscience of Affective Pain and Substance Use Disorder.Alcohol Res. 2021 Dec 16;41(1):14. doi: 10.35946/arcr.v41.1.14. eCollection 2021. Alcohol Res. 2021. PMID: 34976573 Free PMC article. Review.
-
Divergent regulation of distinct glucocorticoid systems in alcohol dependence.Alcohol. 2015 Dec;49(8):811-6. doi: 10.1016/j.alcohol.2015.04.004. Epub 2015 Apr 30. Alcohol. 2015. PMID: 26003866 Free PMC article. Review.
Cited by
-
Alcohol amplifies cingulate cortex signaling and facilitates immobilization-induced hyperalgesia in female rats.Neurosci Lett. 2021 Sep 14;761:136119. doi: 10.1016/j.neulet.2021.136119. Epub 2021 Jul 17. Neurosci Lett. 2021. PMID: 34280506 Free PMC article.
-
Surgical incision pain induced an increase in alcohol consumption in mice.Alcohol. 2024 Jun;117:1-9. doi: 10.1016/j.alcohol.2024.03.005. Epub 2024 Mar 11. Alcohol. 2024. PMID: 38479450
-
Pathophysiological Consequences of At-Risk Alcohol Use; Implications for Comorbidity Risk in Persons Living With Human Immunodeficiency Virus.Front Physiol. 2022 Jan 18;12:758230. doi: 10.3389/fphys.2021.758230. eCollection 2021. Front Physiol. 2022. PMID: 35115952 Free PMC article. Review.
-
Chronic inflammatory pain promotes place preference for fentanyl in male rats but does not change fentanyl self-administration in male and female rats.Neuropharmacology. 2023 Jun 15;231:109512. doi: 10.1016/j.neuropharm.2023.109512. Epub 2023 Mar 21. Neuropharmacology. 2023. PMID: 36948356 Free PMC article.
-
Analgesic effects of alcohol in adults with chronic jaw pain.Alcohol Clin Exp Res. 2022 Aug;46(8):1515-1524. doi: 10.1111/acer.14883. Epub 2022 Aug 22. Alcohol Clin Exp Res. 2022. PMID: 35989585 Free PMC article. Clinical Trial.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

