Protein-N-myristoylation-dependent phosphorylation of serine 13 of tyrosine kinase Lyn by casein kinase 1γ at the Golgi during intracellular protein traffic
- PMID: 33004926
- PMCID: PMC7531007
- DOI: 10.1038/s41598-020-73248-0
Protein-N-myristoylation-dependent phosphorylation of serine 13 of tyrosine kinase Lyn by casein kinase 1γ at the Golgi during intracellular protein traffic
Abstract
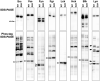
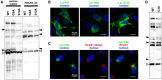
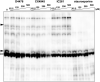
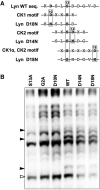
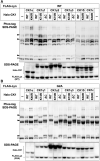
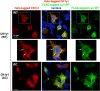
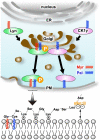
Protein N-myristoylation of Src-family kinases (SFKs) is a critical co-translational modification to anchor the enzymes in the plasma membrane. Phosphorylation of SFKs is also an essential modification for regulating their enzymatic activities. In this study, we used Phos-tag SDS-PAGE to investigate N-myristoylation-dependent phosphorylation of SFKs and their non-N-myristoylated G2A mutants. The serine-13 residue of Lyn (Lyn-S13) was shown to be N-myristoylation-dependently phosphorylated. Although there have been more than 40 reports of mass spectrometric studies on phosphorylation at Lyn-S13, the kinase responsible remained unclear. We succeeded in identifying casein kinase 1γ (CK1γ) as the kinase responsible for phosphorylation of Lyn-S13. In HEK293 cells co-expressing Lyn and CK1γ, the phosphorylation level of Lyn-S13 increased significantly. CK1γ is unique among the CK1 family (α, γ, δ, and ε) in carrying an S-palmitoylation site for membrane binding. Co-expression with the non-S-palmitoylated CK1γ mutant, which localized in the cytosol, gave no increase in the phosphorylation level at Lyn-S13. In HEK293 cells expressing the non-S-palmitoylated Lyn-C3A mutant, on the other hand, the Lyn-C3A mutant was phosphorylated at Lyn-S13, and the mutant remained at the Golgi. These results showed that S-palmitoylated CK1γ can phosphorylate S13 of N-myristoylated Lyn at the Golgi during intracellular protein traffic.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A strategy to identify protein-N-myristoylation-dependent phosphorylation reactions of cellular proteins by using Phos-tag SDS-PAGE.PLoS One. 2019 Nov 21;14(11):e0225510. doi: 10.1371/journal.pone.0225510. eCollection 2019. PLoS One. 2019. PMID: 31751425 Free PMC article.
-
Requirement of the SH4 and tyrosine-kinase domains but not the kinase activity of Lyn for its biosynthetic targeting to caveolin-positive Golgi membranes.Biochim Biophys Acta. 2009 Oct;1790(10):1345-52. doi: 10.1016/j.bbagen.2009.07.009. Epub 2009 Jul 18. Biochim Biophys Acta. 2009. PMID: 19619611
-
Mutants of the protein serine kinase PSKH1 disassemble the Golgi apparatus.Exp Cell Res. 2003 Dec 10;291(2):299-312. doi: 10.1016/j.yexcr.2003.07.009. Exp Cell Res. 2003. PMID: 14644153
-
Phosphorylation of the proteins of the extracellular matrix of mineralized tissues by casein kinase-like activity.Crit Rev Oral Biol Med. 1997;8(4):360-79. doi: 10.1177/10454411970080040101. Crit Rev Oral Biol Med. 1997. PMID: 9391750 Review.
-
Src family kinases: regulation of their activities, levels and identification of new pathways.Biochim Biophys Acta. 2008 Jan;1784(1):56-65. doi: 10.1016/j.bbapap.2007.08.012. Epub 2007 Aug 22. Biochim Biophys Acta. 2008. PMID: 17905674 Review.
Cited by
-
Location, location, location: Protein kinase nanoclustering for optimised signalling output.Elife. 2024 Jan 11;13:e93902. doi: 10.7554/eLife.93902. Elife. 2024. PMID: 38206309 Free PMC article. Review.
-
Characterization of Phosphorylation Status and Kinase Activity of Src Family Kinases Expressed in Cell-Based and Cell-Free Protein Expression Systems.Biomolecules. 2021 Oct 2;11(10):1448. doi: 10.3390/biom11101448. Biomolecules. 2021. PMID: 34680080 Free PMC article.
-
Non-Receptor Tyrosine Kinases: Their Structure and Mechanistic Role in Tumor Progression and Resistance.Cancers (Basel). 2024 Aug 2;16(15):2754. doi: 10.3390/cancers16152754. Cancers (Basel). 2024. PMID: 39123481 Free PMC article. Review.
-
ANKRD22 is an N-myristoylated hairpin-like monotopic membrane protein specifically localized to lipid droplets.Sci Rep. 2021 Sep 28;11(1):19233. doi: 10.1038/s41598-021-98486-8. Sci Rep. 2021. PMID: 34584137 Free PMC article.
-
Protein lipidation in health and disease: molecular basis, physiological function and pathological implication.Signal Transduct Target Ther. 2024 Mar 15;9(1):60. doi: 10.1038/s41392-024-01759-7. Signal Transduct Target Ther. 2024. PMID: 38485938 Free PMC article. Review.
References
-
- Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. - PubMed
-
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. - PubMed
-
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. - PubMed
-
- Ingley E. Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta. 2008;1784:56–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

