Inhibitory Potential of Polyclonal Camel Antibodies against New Delhi Metallo-β-lactamase-1 (NDM-1)
- PMID: 32998307
- PMCID: PMC7584030
- DOI: 10.3390/molecules25194453
Inhibitory Potential of Polyclonal Camel Antibodies against New Delhi Metallo-β-lactamase-1 (NDM-1)
Abstract
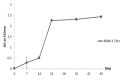
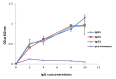
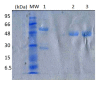
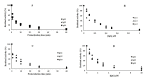
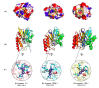
New Delhi Metallo-β-lactamase-1 (NDM-1) is the most prevalent type of metallo-β-lactamase, able to hydrolyze almost all antibiotics of the β-lactam group, leading to multidrug-resistant bacteria. To date, there are no clinically relevant inhibitors to fight NDM-1. The use of dromedary polyclonal antibody inhibitors against NDM-1 represents a promising new class of molecules with inhibitory activity. In the current study, immunoreactivities of dromedary Immunoglobulin G (IgG) isotypes containing heavy-chain and conventional antibodies were tested after successful immunization of dromedary using increasing amounts of the recombinant NDM-1 enzyme. Inhibition kinetic assays, performed using a spectrophotometric method with nitrocefin as a reporter substrate, demonstrated that IgG1, IgG2, and IgG3 were able to inhibit not only the hydrolytic activity of NDM-1 but also Verona integron-encoded metallo-β-lactamase (VIM-1) (subclass B1) and L1 metallo-β-lactamase (L1) (subclass B3) with inhibitory concentration (IC50) values ranging from 100 to 0.04 μM. Investigations on the ability of IgG subclasses to reduce the growth of recombinant Escherichia coli BL21(DE3)/codon plus cells containing the recombinant plasmid expressing NDM-1, L1, or VIM-1 showed that the addition of IgGs (4 and 8 mg/L) to the cell culture was unable to restore the susceptibility of carbapenems. Interestingly, IgGs were able to interact with NDM-1, L1, and VIM-1 when tested on the periplasm extract of each cultured strain. The inhibitory concentration was in the micromolar range for all β-lactams tested. A visualization of the 3D structural basis using the three enzyme Protein Data Bank (PDB) files supports preliminarily the recorded inhibition of the three MBLs.
Keywords: NDM-1; antibiotic resistance; camel antibodies; metallo-β-lactamases; potential inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Novel metallo-β-lactamases inhibitors restore the susceptibility of carbapenems to New Delhi metallo-lactamase-1 (NDM-1)-harbouring bacteria.Br J Pharmacol. 2024 Jan;181(1):54-69. doi: 10.1111/bph.16210. Epub 2023 Sep 6. Br J Pharmacol. 2024. PMID: 37539785
-
In Vitro and In Vivo Development of a β-Lactam-Metallo-β-Lactamase Inhibitor: Targeting Carbapenem-Resistant Enterobacterales.ACS Infect Dis. 2023 Mar 10;9(3):486-496. doi: 10.1021/acsinfecdis.2c00485. Epub 2023 Feb 14. ACS Infect Dis. 2023. PMID: 36786013 Free PMC article.
-
Small Molecule Carboxylates Inhibit Metallo-β-lactamases and Resensitize Carbapenem-Resistant Bacteria to Meropenem.ACS Infect Dis. 2020 Jun 12;6(6):1366-1371. doi: 10.1021/acsinfecdis.9b00459. Epub 2020 Apr 3. ACS Infect Dis. 2020. PMID: 32227874 Free PMC article.
-
A close look onto structural models and primary ligands of metallo-β-lactamases.Drug Resist Updat. 2018 Sep;40:1-12. doi: 10.1016/j.drup.2018.08.001. Epub 2018 Aug 25. Drug Resist Updat. 2018. PMID: 30466711 Free PMC article. Review.
-
Ten Years with New Delhi Metallo-β-lactamase-1 (NDM-1): From Structural Insights to Inhibitor Design.ACS Infect Dis. 2019 Jan 11;5(1):9-34. doi: 10.1021/acsinfecdis.8b00247. Epub 2018 Nov 28. ACS Infect Dis. 2019. PMID: 30421910 Review.
Cited by
-
Development of Peptide-based Metallo-β-lactamase Inhibitors as a New Strategy to Combat Antimicrobial Resistance: A Mini-review.Curr Pharm Des. 2022;28(44):3538-3545. doi: 10.2174/1381612828666220929154255. Curr Pharm Des. 2022. PMID: 36177630 Review.
-
Neutralizing Dromedary-Derived Nanobodies Against BotI-Like Toxin From the Most Hazardous Scorpion Venom in the Middle East and North Africa Region.Front Immunol. 2022 Apr 19;13:863012. doi: 10.3389/fimmu.2022.863012. eCollection 2022. Front Immunol. 2022. PMID: 35514999 Free PMC article.
-
Characterization of rabbit polyclonal antibody against camel recombinant nanobodies.Open Life Sci. 2022 Jun 15;17(1):659-675. doi: 10.1515/biol-2022-0065. eCollection 2022. Open Life Sci. 2022. PMID: 35800073 Free PMC article.
-
Camel-Derived Nanobodies as Potent Inhibitors of New Delhi Metallo-β-Lactamase-1 Enzyme.Molecules. 2024 Mar 22;29(7):1431. doi: 10.3390/molecules29071431. Molecules. 2024. PMID: 38611711 Free PMC article.
References
-
- Bradford P.A. Epidemiology of resistance. In: Fong I.W., Shlaes D., Drlica K., editors. Antimicrobial Resistance and Implications for the 21st Century. 2nd ed. Springer International Publishing; New York, NY, USA: 2018.
-
- Paterson D.L., Bonomo R.A. Multidrug-Resistant Gram-Negative Pathogens: The Urgent Need for ‘Old’ Polymyxins. Adv. Exp. Med. Biol. 2019;1145:9–13. - PubMed
-
- Ambler R.P. The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980;289:321–331. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

