First-in-human Phase 1 open label study of the BET inhibitor ODM-207 in patients with selected solid tumours
- PMID: 32989226
- PMCID: PMC7722752
- DOI: 10.1038/s41416-020-01077-z
First-in-human Phase 1 open label study of the BET inhibitor ODM-207 in patients with selected solid tumours
Abstract
Background: Bromodomain and extra-terminal domain (BET) proteins are reported to be epigenetic anti-cancer drug targets. This first-in-human study evaluated the safety, pharmacokinetics and preliminary anti-tumour activity of the BET inhibitor ODM-207 in patients with selected solid tumours.
Methods: This was an open-label Phase 1 study comprised of a dose escalation part, and evaluation of the effect of food on pharmacokinetics. ODM-207 was administered orally once daily. The dose escalation part was initiated with a dose titration in the initial cohort, followed by a 3 + 3 design.
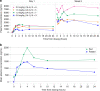
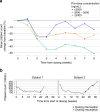
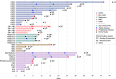
Results: Thirty-five patients were treated with ODM-207, of whom 12 (34%) had castrate-resistant prostate cancer. One dose-limiting toxicity of intolerable fatigue was observed. The highest studied dose achieved was 2 mg/kg due to cumulative toxicity observed beyond the dose-limiting toxicity (DLT) treatment window. Common AEs included thrombocytopenia, asthenia, nausea, anorexia, diarrhoea, fatigue, and vomiting. Platelet count decreased proportionally to exposure with rapid recovery upon treatment discontinuation. No partial or complete responses were observed.
Conclusions: ODM-207 shows increasing exposure in dose escalation and was safe at doses up to 2 mg/kg but had a narrow therapeutic window.
Clinical trial registration: The clinical trial registration number is NCT03035591.
Conflict of interest statement
All authors have completed the Unified Competing Interest form (
Figures
Comment in
-
Bromodomain inhibitors a decade later: a promise unfulfilled?Br J Cancer. 2020 Dec;123(12):1713-1714. doi: 10.1038/s41416-020-01079-x. Epub 2020 Sep 29. Br J Cancer. 2020. PMID: 32989227 Free PMC article.
Similar articles
-
Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial.Lancet Oncol. 2014 Aug;15(9):975-85. doi: 10.1016/S1470-2045(14)70240-2. Epub 2014 Jun 25. Lancet Oncol. 2014. PMID: 24974051 Clinical Trial.
-
Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study.Lancet Oncol. 2019 Oct;20(10):1454-1466. doi: 10.1016/S1470-2045(19)30412-7. Epub 2019 Aug 9. Lancet Oncol. 2019. PMID: 31405822 Clinical Trial.
-
Pharmacokinetics, Antitumor Activity, and Safety of ODM-201 in Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: An Open-label Phase 1 Study.Eur Urol. 2016 May;69(5):834-40. doi: 10.1016/j.eururo.2015.09.046. Epub 2015 Oct 17. Eur Urol. 2016. PMID: 26463318 Clinical Trial.
-
Targeting BET bromodomain proteins in solid tumors.Oncotarget. 2016 Aug 16;7(33):53997-54009. doi: 10.18632/oncotarget.9804. Oncotarget. 2016. PMID: 27283767 Free PMC article. Review.
-
Oral cancer treatment: developments in chemotherapy and beyond.Br J Cancer. 2002 Oct 21;87(9):933-7. doi: 10.1038/sj.bjc.6600591. Br J Cancer. 2002. PMID: 12434279 Free PMC article. Review.
Cited by
-
Sustained Clinical Response to Immunotherapy Followed by BET Inhibitor in a Patient with Unresectable Sinonasal NUT Carcinoma.J Immunother Precis Oncol. 2024 Feb 5;7(1):67-72. doi: 10.36401/JIPO-23-19. eCollection 2024 Feb. J Immunother Precis Oncol. 2024. PMID: 38327754 Free PMC article.
-
BET Proteins as Attractive Targets for Cancer Therapeutics.Int J Mol Sci. 2021 Oct 14;22(20):11102. doi: 10.3390/ijms222011102. Int J Mol Sci. 2021. PMID: 34681760 Free PMC article. Review.
-
Transcriptional super-enhancers control cancer stemness and metastasis genes in squamous cell carcinoma.Nat Commun. 2021 Jun 25;12(1):3974. doi: 10.1038/s41467-021-24137-1. Nat Commun. 2021. PMID: 34172737 Free PMC article.
-
Therapeutic Approaches to Targeting Androgen Receptor Splice Variants.Cells. 2024 Jan 4;13(1):104. doi: 10.3390/cells13010104. Cells. 2024. PMID: 38201308 Free PMC article. Review.
-
Targeting radioresistance and replication fork stability in prostate cancer.JCI Insight. 2022 May 9;7(9):e152955. doi: 10.1172/jci.insight.152955. JCI Insight. 2022. PMID: 35349486 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

