Infection of Human Dental Pulp Stromal Cells by Streptococcus mutans: Shedding Light on Bacteria Pathogenicity and Pulp Inflammation
- PMID: 32984312
- PMCID: PMC7487799
- DOI: 10.3389/fcell.2020.00785
Infection of Human Dental Pulp Stromal Cells by Streptococcus mutans: Shedding Light on Bacteria Pathogenicity and Pulp Inflammation
Abstract
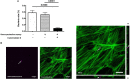
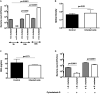
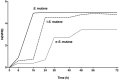
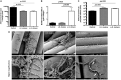
Cariogenic Streptococcus mutans (S. mutans) is implicated in the dental pulp necrosis but also in cardiovascular tissue infections. Herein, the purpose was to elucidate how human dental pulp derived stromal cells (DPSCs) react toward a direct interaction with S. mutans. DPSCs were challenged with S. mutans. Following 3 h of interaction, DPSCs were able to internalize S. mutans (rate < 1%), and F-actin fibers played a significant role in this process. S. mutans persisted in the DPSCs for 48 h without causing a cytotoxic effect. S. mutans was, however, able to get out of the DPSCs cytoplasm and to proliferate in the extracellular environment. Yet, we noticed several adaptive responses of bacteria to the extracellular environment such as a modification of the kinetic growth, the increase in biofilm formation on type I collagen and polyester fabrics, as well as a tolerance toward amoxicillin. In response to infection, DPSCs adopted a proinflammatory profile by increasing the secretion of IL-8, lL-1β, and TNF-α, strengthening the establishment of the dental pulp inflammation. Overall, these findings showed a direct impact of S. mutans on DPSCs, providing new insights into the potential role of S. mutans in infective diseases.
Keywords: S. mutans; dental pulp derived stromal cells; inflammation; internalization; pathogenicity.
Copyright © 2020 Maisonneuve, Chevrier, Dubus, Varin, Sergheraert, Gangloff, Reffuveille, Mauprivez and Kerdjoudj.
Figures
Similar articles
-
TLR4 activation by lipopolysaccharide and Streptococcus mutans induces differential regulation of proliferation and migration in human dental pulp stem cells.J Endod. 2014 Sep;40(9):1375-81. doi: 10.1016/j.joen.2014.03.015. Epub 2014 May 10. J Endod. 2014. PMID: 25146018
-
Can Pulp Fibroblasts Kill Cariogenic Bacteria? Role of Complement Activation.J Dent Res. 2015 Dec;94(12):1765-72. doi: 10.1177/0022034515611074. Epub 2015 Oct 13. J Dent Res. 2015. PMID: 26464397
-
DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis.J Dent Res. 2016 Oct;95(11):1274-81. doi: 10.1177/0022034516657817. Epub 2016 Jul 6. J Dent Res. 2016. PMID: 27384335 Free PMC article.
-
Effects of Antimicrobial Peptide GH12 on the Cariogenic Properties and Composition of a Cariogenic Multispecies Biofilm.Appl Environ Microbiol. 2018 Nov 30;84(24):e01423-18. doi: 10.1128/AEM.01423-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30341079 Free PMC article.
-
Characterisation of dental pulp stem cells: a new horizon for tissue regeneration?Arch Oral Biol. 2012 Nov;57(11):1439-58. doi: 10.1016/j.archoralbio.2012.08.010. Epub 2012 Sep 14. Arch Oral Biol. 2012. PMID: 22981360 Review.
Cited by
-
Bitter Taste Receptor T2R14 Modulates Gram-Positive Bacterial Internalization and Survival in Gingival Epithelial Cells.Int J Mol Sci. 2021 Sep 14;22(18):9920. doi: 10.3390/ijms22189920. Int J Mol Sci. 2021. PMID: 34576085 Free PMC article.
-
Implementing the Design of Experiments (DoE) Concept into the Development of Mucoadhesive Tablets Containing Orange Peel Extract as a Potential Concept for the Treatment of Oral Infections.Materials (Basel). 2024 Oct 28;17(21):5234. doi: 10.3390/ma17215234. Materials (Basel). 2024. PMID: 39517510 Free PMC article.
References
LinkOut - more resources
Full Text Sources

