An optical coherence tomography comparison of coronary arterial plaque calcification in patients with end-stage renal disease and diabetes mellitus
- PMID: 32981349
- PMCID: PMC7919205
- DOI: 10.1177/1479164120958425
An optical coherence tomography comparison of coronary arterial plaque calcification in patients with end-stage renal disease and diabetes mellitus
Abstract
Background: Coronary arterial plaques in patients with end-stage renal disease (ESRD) are assumed to have increased calcification due to underlying renal disease or initiation of dialysis. This relationship may be confounded by comorbid type 2 diabetes mellitus (DM).
Methods: From a single-center OCT registry, 60 patients were analyzed. Twenty patients with ESRD and diabetes (ESRD-DM) were compared to 2 groups of non-ESRD patients: 20 with and 20 without diabetes. In each patient, one 20 mm segment within the culprit vessel was analyzed.
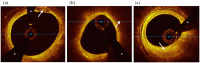
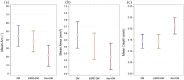
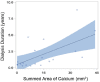
Results: ESRD-DM patients exhibited similar calcium burden, arc, and area compared to patients with diabetes alone. When compared to patients without diabetes, patients with diabetes exhibited a greater summed area of calcium (DM: Median 9.0, IQR [5.3-28] mm2 vs Non-DM: 3.5 [0.1-14] mm2, p = 0.04) and larger calcium deposits by arc (DM: Mean 45 ± SE 6.2° vs Non-DM: 21 ± 6.2°, p = 0.01) and area (DM: 0.58 ± 0.10 mm2 vs Non-DM: 0.26 ± 0.10 mm2, p = 0.03). Calcification deposits in ESRD-DM patients (0.14 ± 0.02 mm) and patients with diabetes (0.14 ± 0.02 mm) were more superficially located relative to patients without diabetes (0.21 ± 0.02 mm), p = 0.01 for both.
Conclusions: Coronary calcification in DM and ESRD-DM groups exhibited similar burden, deposit size, and depth within the arterial wall. The increase in coronary calcification and cardiovascular disease events seen in ESRD-DM patients may not be secondary to ESRD and dialysis, but instead due to a combination of declining renal function and diabetes.
Keywords: Optical coherence tomography; atherosclerosis; calcification; coronary artery calcium; coronary artery disease; diabetes mellitus type 2; end-stage renal disease.
Conflict of interest statement
Figures


Similar articles
-
Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study.Cardiovasc Diabetol. 2017 Dec 1;16(1):152. doi: 10.1186/s12933-017-0635-2. Cardiovasc Diabetol. 2017. PMID: 29195505 Free PMC article.
-
Coronary Plaque Characteristics in Hemodialysis-Dependent Patients as Assessed by Optical Coherence Tomography.Am J Cardiol. 2017 May 1;119(9):1313-1319. doi: 10.1016/j.amjcard.2017.01.022. Epub 2017 Feb 9. Am J Cardiol. 2017. PMID: 28279437
-
Relationships of coronary culprit-plaque characteristics with duration of diabetes mellitus in acute myocardial infarction: an intravascular optical coherence tomography study.Cardiovasc Diabetol. 2019 Oct 19;18(1):136. doi: 10.1186/s12933-019-0944-8. Cardiovasc Diabetol. 2019. PMID: 31629406 Free PMC article.
-
Vascular calcification in end-stage renal disease.J Nephrol. 2002 Nov-Dec;15 Suppl 6:S82-5. J Nephrol. 2002. PMID: 12515378 Review.
-
Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications.Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):205-217. doi: 10.1161/ATVBAHA.116.306258. Epub 2016 Dec 22. Arterioscler Thromb Vasc Biol. 2017. PMID: 28062508 Free PMC article. Review.
Cited by
-
Age-Stratified Prevalence and Relative Prognostic Significance of Traditional Atherosclerotic Risk Factors: A Report from the Nationwide Registry of Percutaneous Coronary Interventions in Japan.J Am Heart Assoc. 2023 Nov 7;12(21):e030881. doi: 10.1161/JAHA.123.030881. Epub 2023 Oct 18. J Am Heart Assoc. 2023. PMID: 37850459 Free PMC article.
-
A Review Paper on Optical Coherence Tomography Evaluation of Coronary Calcification Pattern: Is It Relevant Today?J Cardiovasc Dev Dis. 2024 Jul 24;11(8):231. doi: 10.3390/jcdd11080231. J Cardiovasc Dev Dis. 2024. PMID: 39195139 Free PMC article. Review.
References
-
- United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018.
-
- Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 1999; 10(7): 1606–1615. - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al.. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382(9889): 339–352. - PubMed
-
- Go AS, Chertow GM, Fan D, et al.. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351(13): 1296–1305. - PubMed
-
- Nakano T, Ninomiya T, Sumiyoshi S, et al.. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis 2010; 55(1): 21–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

