Picky ABCG5/G8 and promiscuous ABCG2 - a tale of fatty diets and drug toxicity
- PMID: 32978801
- PMCID: PMC7756502
- DOI: 10.1002/1873-3468.13938
Picky ABCG5/G8 and promiscuous ABCG2 - a tale of fatty diets and drug toxicity
Abstract
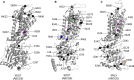
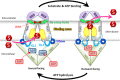
Structural data on ABCG5/G8 and ABCG2 reveal a unique molecular architecture for subfamily G ATP-binding cassette (ABCG) transporters and disclose putative substrate-binding sites. ABCG5/G8 and ABCG2 appear to use several unique structural motifs to execute transport, including the triple helical bundles, the membrane-embedded polar relay, the re-entry helices, and a hydrophobic valve. Interestingly, ABCG2 shows extreme substrate promiscuity, whereas ABCG5/G8 transports only sterol molecules. ABCG2 structures suggest a large internal cavity, serving as a binding region for substrates and inhibitors, while mutational and pharmacological analyses support the notion of multiple binding sites. By contrast, ABCG5/G8 shows a collapsed cavity of insufficient size to hold substrates. Indeed, mutational analyses indicate a sterol-binding site at the hydrophobic interface between the transporter and the lipid bilayer. In this review, we highlight key differences and similarities between ABCG2 and ABCG5/G8 structures. We further discuss the relevance of distinct and shared structural features in the context of their physiological functions. Finally, we elaborate on how ABCG2 and ABCG5/G8 could pave the way for studies on other ABCG transporters.
Keywords: ABCG2; ABCG5; ABCG8; ATP-binding cassette; cholesterol efflux; membranes; multidrug resistance; polar relay; structural biology.
© The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures


Similar articles
-
Transmembrane Polar Relay Drives the Allosteric Regulation for ABCG5/G8 Sterol Transporter.Int J Mol Sci. 2020 Nov 19;21(22):8747. doi: 10.3390/ijms21228747. Int J Mol Sci. 2020. PMID: 33228147 Free PMC article.
-
Snapshots of ABCG1 and ABCG5/G8: A Sterol's Journey to Cross the Cellular Membranes.Int J Mol Sci. 2022 Dec 28;24(1):484. doi: 10.3390/ijms24010484. Int J Mol Sci. 2022. PMID: 36613930 Free PMC article. Review.
-
Structure of the Human Cholesterol Transporter ABCG1.J Mol Biol. 2021 Oct 15;433(21):167218. doi: 10.1016/j.jmb.2021.167218. Epub 2021 Aug 28. J Mol Biol. 2021. PMID: 34461069
-
Association of ABCG5 and ABCG8 Transporters with Sitosterolemia.Adv Exp Med Biol. 2024;1440:31-42. doi: 10.1007/978-3-031-43883-7_2. Adv Exp Med Biol. 2024. PMID: 38036873 Review.
-
Metformin and AMP Kinase Activation Increase Expression of the Sterol Transporters ABCG5/8 (ATP-Binding Cassette Transporter G5/G8) With Potential Antiatherogenic Consequences.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1493-1503. doi: 10.1161/ATVBAHA.118.311212. Epub 2018 May 31. Arterioscler Thromb Vasc Biol. 2018. PMID: 29853564 Free PMC article.
Cited by
-
Identification of genomic regions associated with fatty acid metabolism across blood, liver, backfat and muscle in pigs.Genet Sel Evol. 2024 Sep 26;56(1):66. doi: 10.1186/s12711-024-00933-3. Genet Sel Evol. 2024. PMID: 39327557 Free PMC article.
-
Structure and dynamics of cholesterol-mediated aquaporin-0 arrays and implications for lipid rafts.Elife. 2024 Sep 2;12:RP90851. doi: 10.7554/eLife.90851. Elife. 2024. PMID: 39222068 Free PMC article.
-
Multidrug Resistance in Mammals and Fungi-From MDR to PDR: A Rocky Road from Atomic Structures to Transport Mechanisms.Int J Mol Sci. 2021 Apr 30;22(9):4806. doi: 10.3390/ijms22094806. Int J Mol Sci. 2021. PMID: 33946618 Free PMC article. Review.
-
The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis.Int J Mol Sci. 2021 Jun 23;22(13):6711. doi: 10.3390/ijms22136711. Int J Mol Sci. 2021. PMID: 34201488 Free PMC article. Review.
-
Transmembrane Polar Relay Drives the Allosteric Regulation for ABCG5/G8 Sterol Transporter.Int J Mol Sci. 2020 Nov 19;21(22):8747. doi: 10.3390/ijms21228747. Int J Mol Sci. 2020. PMID: 33228147 Free PMC article.
References
-
- Kuchler K (2011) The ABC of ABCs: multidrug resistance and genetic diseases. FEBS J 278, 3189. - PubMed
-
- Locher KP (2016) Mechanistic diversity in ATP‐binding cassette (ABC) transporters. Nat Struct Mol Biol 23, 487–493. - PubMed
-
- Ernst R, Kueppers P, Stindt J, Kuchler K and Schmitt L (2010) Multidrug efflux pumps: substrate selection in ATP‐binding cassette multidrug efflux pumps–first come, first served? FEBS J 277, 540–549. - PubMed
-
- Kohut P, Wüstner D, Hronska L, Kuchler K, Hapala I and Valachovic M (2011) The role of ABC proteins Aus1p and Pdr11p in the uptake of external sterols in yeast: dehydroergosterol fluorescence study. Biochem Biophys Res Commun 404, 233–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

