Membrane Tension Can Enhance Adaptation to Maintain Polarity of Migrating Cells
- PMID: 32976760
- PMCID: PMC7642449
- DOI: 10.1016/j.bpj.2020.08.035
Membrane Tension Can Enhance Adaptation to Maintain Polarity of Migrating Cells
Abstract
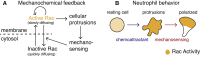
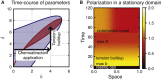
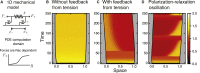
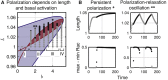
Migratory cells are known to adapt to environments that contain wide-ranging levels of chemoattractant. Although biochemical models of adaptation have been previously proposed, here, we discuss a different mechanism based on mechanosensing, in which the interaction between biochemical signaling and cell tension facilitates adaptation. We describe and analyze a model of mechanochemical-based adaptation coupling a mechanics-based physical model of cell tension coupled with the wave-pinning reaction-diffusion model for Rac GTPase activity. The mathematical analysis of this model, simulations of a simplified one-dimensional cell geometry, and two-dimensional finite element simulations of deforming cells reveal that as a cell protrudes under the influence of high stimulation levels, tension-mediated inhibition of Rac signaling causes the cell to polarize even when initially overstimulated. Specifically, tension-mediated inhibition of Rac activation, which has been experimentally observed in recent years, facilitates this adaptation by countering the high levels of environmental stimulation. These results demonstrate how tension-related mechanosensing may provide an alternative (and potentially complementary) mechanism for cell adaptation.
Copyright © 2020. Published by Elsevier Inc.
Figures


Similar articles
-
Cell Size, Mechanical Tension, and GTPase Signaling in the Single Cell.Bull Math Biol. 2020 Feb 3;82(2):28. doi: 10.1007/s11538-020-00702-5. Bull Math Biol. 2020. PMID: 32016583
-
Exploring the inhibitory effect of membrane tension on cell polarization.PLoS Comput Biol. 2017 Jan 30;13(1):e1005354. doi: 10.1371/journal.pcbi.1005354. eCollection 2017 Jan. PLoS Comput Biol. 2017. PMID: 28135277 Free PMC article.
-
A mechanochemical model for rho GTPase mediated cell polarization.J Theor Biol. 2020 Nov 7;504:110386. doi: 10.1016/j.jtbi.2020.110386. Epub 2020 Jul 9. J Theor Biol. 2020. PMID: 32653321
-
Physical model of cellular symmetry breaking.Cold Spring Harb Perspect Biol. 2009 Jul;1(1):a001909. doi: 10.1101/cshperspect.a001909. Cold Spring Harb Perspect Biol. 2009. PMID: 20066077 Free PMC article. Review.
-
Cell polarity: mechanochemical patterning.Trends Cell Biol. 2013 Feb;23(2):72-80. doi: 10.1016/j.tcb.2012.10.009. Epub 2012 Nov 23. Trends Cell Biol. 2013. PMID: 23182746 Review.
Cited by
-
Understanding the interplay of membrane trafficking, cell surface mechanics, and stem cell differentiation.Semin Cell Dev Biol. 2023 Jan 15;133:123-134. doi: 10.1016/j.semcdb.2022.05.010. Epub 2022 May 28. Semin Cell Dev Biol. 2023. PMID: 35641408 Free PMC article. Review.
-
Three-dimensional stochastic simulation of chemoattractant-mediated excitability in cells.PLoS Comput Biol. 2021 Jul 14;17(7):e1008803. doi: 10.1371/journal.pcbi.1008803. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34260581 Free PMC article.
-
Pannexin Channel Regulation of Cell Migration: Focus on Immune Cells.Front Immunol. 2021 Dec 16;12:750480. doi: 10.3389/fimmu.2021.750480. eCollection 2021. Front Immunol. 2021. PMID: 34975840 Free PMC article. Review.
-
Cortical tension initiates the positive feedback loop between cadherin and F-actin.Biophys J. 2022 Feb 15;121(4):596-606. doi: 10.1016/j.bpj.2022.01.006. Epub 2022 Jan 11. Biophys J. 2022. PMID: 35031276 Free PMC article.
-
A free boundary mechanobiological model of epithelial tissues.Proc Math Phys Eng Sci. 2020 Nov;476(2243):20200528. doi: 10.1098/rspa.2020.0528. Epub 2020 Nov 18. Proc Math Phys Eng Sci. 2020. PMID: 33362419 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

