Human amniotic epithelial cells ameliorate kidney damage in ischemia-reperfusion mouse model of acute kidney injury
- PMID: 32967729
- PMCID: PMC7510147
- DOI: 10.1186/s13287-020-01917-y
Human amniotic epithelial cells ameliorate kidney damage in ischemia-reperfusion mouse model of acute kidney injury
Abstract
Background: Acute kidney injury (AKI) is a common clinical disease with complex pathophysiology and limited therapeutic choices. This prompts the need for novel therapy targeting multiple aspects of this disease. Human amnion epithelial cell (hAEC) is an ideal stem cell source. Increasing evidence suggests that exosomes may act as critical cell-cell communicators. Accordingly, we assessed the therapeutic potential of hAECs and their derived exosomes (hAECs-EXO) in ischemia reperfusion mouse model of AKI and explored the underlying mechanisms.
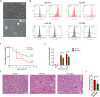
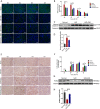
Methods: The hAECs were primary cultured, and hAECs-EXO were isolated and characterized. An ischemic-reperfusion injury-induced AKI (IRI-AKI) mouse model was established to mimic clinical ischemic kidney injury with different disease severity. Mouse blood creatinine level was used to assess renal function, and kidney specimens were processed to detect cell proliferation, apoptosis, and capillary density. Macrophage infiltration was analyzed by flow cytometry. hAEC-derived exosomes (hAECs-EXO) were used to treat hypoxia-reoxygenation (H/R) injured HK-2 cells and mouse bone marrow-derived macrophages to evaluate their protective effect in vitro. Furthermore, hAECs-EXO were subjected to liquid chromatography-tandem mass spectrometry for proteomic profiling.
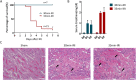
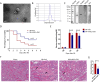
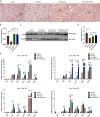
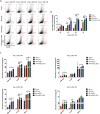
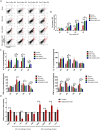
Results: We found that systematically administered hAECs could improve mortality and renal function in IRI-AKI mice, decrease the number of apoptotic cells, prevent peritubular capillary loss, and modulate kidney local immune response. However, hAECs showed very low kidney tissue integration. Exosomes isolated from hAECs recapitulated the renal protective effects of their source cells. In vitro, hAECs-EXO protected HK-2 cells from H/R injury-induced apoptosis and promoted bone marrow-derived macrophage polarization toward M2 phenotype. Proteomic analysis on hAECs-EXO revealed proteins involved in extracellular matrix organization, growth factor signaling pathways, cytokine production, and immunomodulation. These findings demonstrated that paracrine of exosomes might be the key mechanism of hAECs in alleviating renal ischemia reperfusion injury.
Conclusions: We reported hAECs could improve survival and ameliorate renal injury in mice with IRI-AKI. The anti-apoptotic, pro-angiogenetic, and immunomodulatory capabilities of hAECs are at least partially, through paracrine pathways. hAECs-EXO might be a promising clinical therapeutic tool, overcoming the weaknesses and risks associated with the use of native stem cells, for patients with AKI.
Keywords: Acute kidney injury; Cell therapy; Exosome; Human amnion epithelial cells; Ischemia.
Conflict of interest statement
Li Yang has a patent pending for the use of hAECs in repair of the injured kidney after AKI. Shanghai iCELL Biotechnology company declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The other authors have no financial conflicts of interest.
Figures
Similar articles
-
Exosome from indoleamine 2,3-dioxygenase-overexpressing bone marrow mesenchymal stem cells accelerates repair process of ischemia/reperfusion-induced acute kidney injury by regulating macrophages polarization.Stem Cell Res Ther. 2022 Jul 28;13(1):367. doi: 10.1186/s13287-022-03075-9. Stem Cell Res Ther. 2022. PMID: 35902956 Free PMC article.
-
Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis.Stem Cell Res Ther. 2022 Jul 15;13(1):297. doi: 10.1186/s13287-022-02986-x. Stem Cell Res Ther. 2022. PMID: 35841017 Free PMC article.
-
Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1.Theranostics. 2020 Jul 25;10(21):9561-9578. doi: 10.7150/thno.42153. eCollection 2020. Theranostics. 2020. PMID: 32863945 Free PMC article.
-
[The role of macrophage polarization and interaction with renal tubular epithelial cells in ischemia-reperfusion induced acute kidney injury].Sheng Li Xue Bao. 2022 Feb 25;74(1):28-38. Sheng Li Xue Bao. 2022. PMID: 35199123 Review. Chinese.
-
The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury.Int J Mol Sci. 2019 Jul 19;20(14):3529. doi: 10.3390/ijms20143529. Int J Mol Sci. 2019. PMID: 31330934 Free PMC article. Review.
Cited by
-
Stem cell-based therapy for ameliorating intrauterine adhesion and endometrium injury.Stem Cell Res Ther. 2021 Oct 30;12(1):556. doi: 10.1186/s13287-021-02620-2. Stem Cell Res Ther. 2021. PMID: 34717746 Free PMC article. Review.
-
The therapeutic potential of exosomes in lung cancer.Cell Oncol (Dordr). 2023 Oct;46(5):1181-1212. doi: 10.1007/s13402-023-00815-8. Epub 2023 May 11. Cell Oncol (Dordr). 2023. PMID: 37365450 Review.
-
Exosomes Highlight Future Directions in the Treatment of Acute Kidney Injury.Int J Mol Sci. 2023 Oct 25;24(21):15568. doi: 10.3390/ijms242115568. Int J Mol Sci. 2023. PMID: 37958550 Free PMC article. Review.
-
Therapeutic Potential of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Prevention of Organ Injuries Induced by Traumatic Hemorrhagic Shock.Front Immunol. 2021 Sep 29;12:749659. doi: 10.3389/fimmu.2021.749659. eCollection 2021. Front Immunol. 2021. PMID: 34659252 Free PMC article. Review.
-
Human Amnion Epithelial Cells and Their Derived Exosomes Alleviate Sepsis-Associated Acute Kidney Injury via Mitigating Endothelial Dysfunction.Front Med (Lausanne). 2022 Mar 24;9:829606. doi: 10.3389/fmed.2022.829606. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35402422 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

