Adipocytes: active facilitators in epithelial ovarian cancer progression?
- PMID: 32967712
- PMCID: PMC7513299
- DOI: 10.1186/s13048-020-00718-4
Adipocytes: active facilitators in epithelial ovarian cancer progression?
Abstract
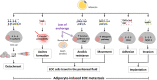
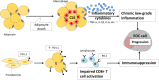
There is growing evidence that adipocytes play important roles in the progression of multiple cancers. Moreover, in obesity, adipocytes alter their original functions and contribute to the metabolic and inflammatory changes of adipose tissue microenvironment, which can further enhance tumor development. At present, the roles of adipocytes in the pathogenesis of epithelial ovarian cancer (EOC) are far from being fully elucidated. Herein, we summarized the recent advances in understanding the roles of adipocytes in EOC progression. Adipocytes, close neighbors of EOC tissue, promote EOC growth, invasion, metastasis and angiogenesis through adipokine secretion, metabolic remodeling and immune microenvironment modulation. Moreover, adipocytes are important therapeutic targets and may work as useful anticancer drug delivery depot for EOC treatment. Furthermore, adipocytes also act as a therapeutic obstacle for their involvement in EOC treatment resistance. Hence, better characterization of the adipocytes in EOC microenvironment and the crosstalk between adipocytes and EOC cells may provide insights into EOC progression and suggest novel therapeutic opportunities.
Keywords: Adipocytes; Cancer progression; Epithelial ovarian cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Adipocyte derived exosomes promote cell invasion and challenge paclitaxel efficacy in ovarian cancer.Cell Commun Signal. 2024 Sep 16;22(1):443. doi: 10.1186/s12964-024-01806-4. Cell Commun Signal. 2024. PMID: 39285292 Free PMC article.
-
Activation of SphK1 by adipocytes mediates epithelial ovarian cancer cell proliferation.J Ovarian Res. 2021 May 1;14(1):62. doi: 10.1186/s13048-021-00815-y. J Ovarian Res. 2021. PMID: 33931106 Free PMC article.
-
TRIP13 promotes proliferation and invasion of epithelial ovarian cancer cells through Notch signaling pathway.Eur Rev Med Pharmacol Sci. 2019 Jan;23(2):522-529. doi: 10.26355/eurrev_201901_16864. Eur Rev Med Pharmacol Sci. 2019. PMID: 30720159
-
Adipocyte Microenvironment in Ovarian Cancer: A Critical Contributor?Int J Mol Sci. 2023 Nov 22;24(23):16589. doi: 10.3390/ijms242316589. Int J Mol Sci. 2023. PMID: 38068912 Free PMC article. Review.
-
Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles.Cell Oncol (Dordr). 2020 Aug;43(4):515-538. doi: 10.1007/s13402-020-00513-9. Epub 2020 May 16. Cell Oncol (Dordr). 2020. PMID: 32418122 Review.
Cited by
-
High visceral fat-to-muscle ratio is an independent factor that predicts worse overall survival in patients with primary epithelial ovarian, fallopian tube, and peritoneal cancer.J Ovarian Res. 2023 Jan 21;16(1):19. doi: 10.1186/s13048-023-01098-1. J Ovarian Res. 2023. PMID: 36681847 Free PMC article.
-
Association of Overweight and Inflammatory Indicators with Breast Cancer: A Cross-Sectional Study in Chinese Women.Int J Womens Health. 2024 May 6;16:783-795. doi: 10.2147/IJWH.S428696. eCollection 2024. Int J Womens Health. 2024. PMID: 38737496 Free PMC article.
-
Irisin/FNDC5 inhibits the epithelial-mesenchymal transition of epithelial ovarian cancer cells via the PI3K/Akt pathway.Arch Gynecol Obstet. 2022 Sep;306(3):841-850. doi: 10.1007/s00404-022-06427-1. Epub 2022 Feb 14. Arch Gynecol Obstet. 2022. PMID: 35156135
-
Adipocyte‑rich microenvironment promotes chemoresistance via upregulation of peroxisome proliferator‑activated receptor gamma/ABCG2 in epithelial ovarian cancer.Int J Mol Med. 2024 Apr;53(4):37. doi: 10.3892/ijmm.2024.5361. Epub 2024 Mar 1. Int J Mol Med. 2024. PMID: 38426604 Free PMC article.
-
Etiopathogenesis of ovarian cancer. An inflamm-aging entity?Gynecol Oncol Rep. 2022 Jun 11;42:101018. doi: 10.1016/j.gore.2022.101018. eCollection 2022 Aug. Gynecol Oncol Rep. 2022. PMID: 35719320 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

