Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKKɛ complex inhibitor BX795
- PMID: 32967676
- PMCID: PMC7510327
- DOI: 10.1186/s12967-020-02526-2
Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKKɛ complex inhibitor BX795
Abstract
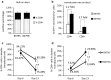
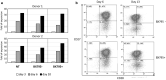
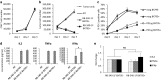
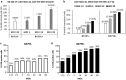
Background: Adoptive transfer of engineered immune cells is a promising strategy for cancer treatment. However, low transduction efficiency particularly when large payload lentiviral vectors are used on primary T cells is a limitation for the development of cell therapy platforms that include multiple constructs bearing long DNA sequences. RB-340-1 is a new CAR T cell that combines two strategies in one product through a CRISPR interference (CRISPRi) circuit. Because multiple regulatory components are included in the circuit, RB-340-1 production needs delivery of two lentiviral vectors into human primary T cells, both containing long DNA sequences. To improve lentiviral transduction efficiency, we looked for inhibitors of receptors involved in antiviral response. BX795 is a pharmacological inhibitor of the TBK1/IKKɛ complex, which has been reported to augment lentiviral transduction of human NK cells and some cell lines, but it has not been tested with human primary T cells. The purpose of this study was to test if BX795 treatment promotes large payload RB-340-1 lentiviral transduction of human primary T cells.
Methods: To make the detection of gene delivery more convenient, we constructed another set of RB-340-1 constructs containing fluorescent labels named RB-340-1F. We incorporated BX795 treatment into the human primary T cell transduction procedure that was optimized for RB-340-1F. We tested BX795 with T cells collected from multiple donors, and detected the effect of BX795 on T cell transduction, phenotype, cell growth and cell function.
Results: We found that BX795 promotes RB-340-1F lentiviral transduction of human primary T cells, without dramatic change in cell growth and T cell functions. Meanwhile, BX795 treatment increased CD8+ T cell ratios in transduced T cells.
Conclusions: These results indicate that BX795 treatment is effective, and might be a safe approach to promote RB-340-1F lentiviral transduction of human primary T cells. This approach might also be helpful for other T cell therapy products that need delivery of complicated platform via large payload lentiviral vectors.
Keywords: BX795; CAR-T; CRISPRi; Lentiviral transduction; T cell engineering.
Conflict of interest statement
The following patent US#9,856,497 relates to this work. Lei S. Qi is a co-founder and scientific advisor of Refuge Biotechnologies.
Figures


Similar articles
-
Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: implications for gene therapy.Hum Gene Ther. 2012 Oct;23(10):1090-100. doi: 10.1089/hum.2012.080. Epub 2012 Sep 10. Hum Gene Ther. 2012. PMID: 22779406 Free PMC article.
-
High Cytotoxic Efficiency of Lentivirally and Alpharetrovirally Engineered CD19-Specific Chimeric Antigen Receptor Natural Killer Cells Against Acute Lymphoblastic Leukemia.Front Immunol. 2020 Jan 24;10:3123. doi: 10.3389/fimmu.2019.03123. eCollection 2019. Front Immunol. 2020. PMID: 32117200 Free PMC article.
-
BX795, a TBK1 inhibitor, exhibits antitumor activity in human oral squamous cell carcinoma through apoptosis induction and mitotic phase arrest.Eur J Pharmacol. 2015 Dec 15;769:287-96. doi: 10.1016/j.ejphar.2015.11.032. Epub 2015 Nov 27. Eur J Pharmacol. 2015. PMID: 26607461
-
Clinical use of lentiviral vectors.Leukemia. 2018 Jul;32(7):1529-1541. doi: 10.1038/s41375-018-0106-0. Epub 2018 Mar 22. Leukemia. 2018. PMID: 29654266 Free PMC article. Review.
-
Toward integrase defective lentiviral vectors for genetic immunization.Curr HIV Res. 2010 Jun;8(4):274-81. doi: 10.2174/157016210791208622. Curr HIV Res. 2010. PMID: 20353396 Review.
Cited by
-
Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy.Leukemia. 2024 Oct 25. doi: 10.1038/s41375-024-02444-y. Online ahead of print. Leukemia. 2024. PMID: 39455854 Review.
-
Engineered CD4 T cells for in vivo delivery of therapeutic proteins.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2318687121. doi: 10.1073/pnas.2318687121. Epub 2024 Sep 23. Proc Natl Acad Sci U S A. 2024. PMID: 39312667
-
Primary T-cell-based delivery platform for in vivo synthesis of engineered proteins.Bioeng Transl Med. 2023 Oct 7;9(1):e10605. doi: 10.1002/btm2.10605. eCollection 2024 Jan. Bioeng Transl Med. 2023. PMID: 38193126 Free PMC article.
-
Chimeric antigen receptor engineered natural killer cells for cancer therapy.Exp Hematol Oncol. 2023 Aug 10;12(1):70. doi: 10.1186/s40164-023-00431-0. Exp Hematol Oncol. 2023. PMID: 37563648 Free PMC article. Review.
-
Nanoscale, antigen encounter-dependent, IL-12 delivery by CAR T cells plus PD-L1 blockade for cancer treatment.J Transl Med. 2023 Feb 28;21(1):158. doi: 10.1186/s12967-023-04014-9. J Transl Med. 2023. PMID: 36855120 Free PMC article.
References
-
- Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–1506. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

