Interferon-beta enhances sensitivity to gemcitabine in pancreatic cancer
- PMID: 32967656
- PMCID: PMC7513525
- DOI: 10.1186/s12885-020-07420-0
Interferon-beta enhances sensitivity to gemcitabine in pancreatic cancer
Abstract
Background: Adjuvant gemcitabine for pancreatic cancer has limited efficacy in the clinical setting. Impaired drug metabolism is associated with treatment resistance. We aimed to evaluate the chemosensitising effect of interferon-beta (IFN-β).
Methods: BxPC-3, CFPAC-1, and Panc-1 cells were pre-treated with IFN-β followed by gemcitabine monotherapy. The effect on cell growth, colony formation, and cell cycle was determined. RT-qPCR was used to measure gene expression. BxPC-3 cells were used in a heterotopic subcutaneous mouse model.
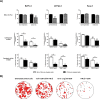
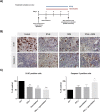
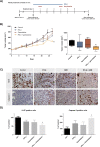
Results: IFN-β increased sensitivity to gemcitabine (4-, 7.7-, and 1.7-fold EC50 decrease in BxPC-3, CFPAC-1, and Panc-1, respectively; all P < 0.001). Findings were confirmed when assessing colony formation. The percentage of cells in the S-phase was significantly increased after IFN-β treatment only in BxPC-3 and CFPAC-1 by 12 and 7%, respectively (p < 0.001 and p < 0.05, respectively). Thereby, IFN-β upregulated expression of the drug transporters SLC28A1 in BxPC-3 (252%) and SLC28A3 in BxPC-3 (127%) and CFPAC-1 (223%) (all p < 0.001). In vivo, combination therapy reduced tumor volume with 45% (P = 0.01). Both ex vivo and in vivo data demonstrate a significant reduction in the number of proliferating cells, whereas apoptosis was increased.
Conclusions: For the first time, we validated the chemosensitising effects of IFN-β when combined with gemcitabine in vitro, ex vivo, and in vivo. This was driven by cell cycle modulation and associated with an upregulation of genes involving intracellular uptake of gemcitabine. The use of IFN-β in combination with gemcitabine seems promising in patients with pancreatic cancer and needs to be further explored.
Keywords: Chemosensitising effect; Drug transporters; Gemcitabine; Interferon-beta; Pancreatic cancer.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

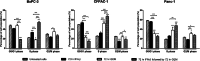

Similar articles
-
OSI-027 inhibits pancreatic ductal adenocarcinoma cell proliferation and enhances the therapeutic effect of gemcitabine both in vitro and in vivo.Oncotarget. 2015 Sep 22;6(28):26230-41. doi: 10.18632/oncotarget.4579. Oncotarget. 2015. PMID: 26213847 Free PMC article.
-
Synergistic antitumor effect of interferon-ß with gemcitabine in interferon-α-non-responsive pancreatic cancer cells.Int J Oncol. 2011 May;38(5):1237-43. doi: 10.3892/ijo.2011.954. Epub 2011 Feb 23. Int J Oncol. 2011. PMID: 21347515
-
RC-3095, a gastrin-releasing peptide receptor antagonist, synergizes with gemcitabine to inhibit the growth of human pancreatic cancer CFPAC-1 in vitro and in vivo.Pancreas. 2014 Jan;43(1):15-21. doi: 10.1097/MPA.0b013e3182a714cf. Pancreas. 2014. PMID: 24326363
-
Silencing pancreatic adenocarcinoma upregulated factor (PAUF) increases the sensitivity of pancreatic cancer cells to gemcitabine.Tumour Biol. 2016 Jun;37(6):7555-64. doi: 10.1007/s13277-015-4641-2. Epub 2015 Dec 18. Tumour Biol. 2016. PMID: 26684804
-
[Gene therapy of human pancreatic cancer cells by human interferon-beta gene].Nihon Rinsho. 2006 Jan;64 Suppl 1:228-31. Nihon Rinsho. 2006. PMID: 16457256 Review. Japanese. No abstract available.
Cited by
-
Revisiting Circulating Extracellular Matrix Fragments as Disease Markers in Myelofibrosis and Related Neoplasms.Cancers (Basel). 2023 Aug 29;15(17):4323. doi: 10.3390/cancers15174323. Cancers (Basel). 2023. PMID: 37686599 Free PMC article. Review.
-
Considerations in the Design of Non-Clinical Development Programmes to Support Non-Viral Genetically Modified Mesenchymal Stromal Cell Therapies.Pharmaceutics. 2021 Jun 2;13(6):823. doi: 10.3390/pharmaceutics13060823. Pharmaceutics. 2021. PMID: 34199356 Free PMC article. Review.
-
Association between Inflammation and Function of Cell Adhesion Molecules Influence on Gastrointestinal Cancer Development.Cells. 2021 Jan 4;10(1):67. doi: 10.3390/cells10010067. Cells. 2021. PMID: 33406733 Free PMC article. Review.
-
The Class I HDAC Inhibitor Valproic Acid Strongly Potentiates Gemcitabine Efficacy in Pancreatic Cancer by Immune System Activation.Biomedicines. 2022 Feb 22;10(3):517. doi: 10.3390/biomedicines10030517. Biomedicines. 2022. PMID: 35327319 Free PMC article.
-
Pre-treatment inflamed tumor immune microenvironment is associated with FOLFIRINOX response in pancreatic cancer.Front Oncol. 2023 Nov 23;13:1274783. doi: 10.3389/fonc.2023.1274783. eCollection 2023. Front Oncol. 2023. PMID: 38074633 Free PMC article.
References
-
- Tehfe M, Dowden S, Kennecke H, El-Maraghi R, Lesperance B, Couture F, et al. Nab-paclitaxel plus gemcitabine versus gemcitabine in patients with metastatic pancreatic adenocarcinoma: Canadian subgroup analysis of the phase 3 MPACT trial. Adv Ther. 2016;33(5):747–759. doi: 10.1007/s12325-016-0327-4. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

