Coordinated Viral Control by Cytotoxic Lymphocytes Ensures Optimal Adaptive NK Cell Responses
- PMID: 32966792
- PMCID: PMC7532550
- DOI: 10.1016/j.celrep.2020.108186
Coordinated Viral Control by Cytotoxic Lymphocytes Ensures Optimal Adaptive NK Cell Responses
Abstract
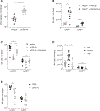
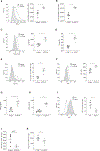
Natural killer (NK) cells play a critical role in controlling viral infections, coordinating the response of innate and adaptive immune systems. They also possess certain features of adaptive lymphocytes, such as undergoing clonal proliferation. However, it is not known whether this adaptive NK cell response can be modulated by other lymphocytes during viral exposure. Here, we show that the clonal expansion of NK cells during mouse cytomegalovirus infection is severely blunted in the absence of cytotoxic CD8+ T cells. This correlates with higher viral burden and an increased pro-inflammatory milieu, which maintains NK cells in a hyper-activated state. Antiviral therapy rescues NK cell expansion in the absence of CD8+ T cells, suggesting that high viral loads have detrimental effects on adaptive NK cell responses. Altogether, our data support a mechanism whereby cytotoxic innate and adaptive lymphocytes cooperate to ensure viral clearance and the establishment of robust clonal NK cell responses.
Keywords: CD8(+) T cell; MCMV; antivirals; cooperation; inflammation; natural killer cell.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


Similar articles
-
Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection.Med Microbiol Immunol. 2012 Nov;201(4):487-95. doi: 10.1007/s00430-012-0263-0. Epub 2012 Sep 11. Med Microbiol Immunol. 2012. PMID: 22965169 Free PMC article. Review.
-
NK cell and Th17 responses are differentially induced in murine cytomegalovirus infected renal allografts and vary according to recipient virus dose and strain.Am J Transplant. 2018 Nov;18(11):2647-2662. doi: 10.1111/ajt.14868. Epub 2018 May 7. Am J Transplant. 2018. PMID: 29659179 Free PMC article.
-
Mouse cytomegalovirus infection overrules T regulatory cell suppression on natural killer cells.Virol J. 2014 Aug 9;11:145. doi: 10.1186/1743-422X-11-145. Virol J. 2014. PMID: 25108672 Free PMC article.
-
Caspase-8 restricts natural killer cell accumulation during MCMV Infection.Med Microbiol Immunol. 2019 Aug;208(3-4):543-554. doi: 10.1007/s00430-019-00617-6. Epub 2019 May 21. Med Microbiol Immunol. 2019. PMID: 31115653 Free PMC article.
-
Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection.Med Microbiol Immunol. 2019 Aug;208(3-4):555-571. doi: 10.1007/s00430-019-00616-7. Epub 2019 May 16. Med Microbiol Immunol. 2019. PMID: 31098689 Review.
Cited by
-
NK- and T-cell repertoire is established early after allogeneic HSCT and is imprinted by CMV reactivation.Blood Adv. 2024 Nov 12;8(21):5612-5624. doi: 10.1182/bloodadvances.2024013117. Blood Adv. 2024. PMID: 39047210 Free PMC article.
-
Decitabine Treatment Induces a Viral Mimicry Response in Cervical Cancer Cells and Further Sensitizes Cells to Chemotherapy.Int J Mol Sci. 2022 Nov 14;23(22):14042. doi: 10.3390/ijms232214042. Int J Mol Sci. 2022. PMID: 36430521 Free PMC article.
References
-
- Arase H, Mocarski ES, Campbell AE, Hill AB, and Lanier LL (2002). Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326. - PubMed
-
- Biron CA, Byron KS, and Sullivan JL (1989). Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

