TILs and Anti-PD1 Therapy: An Alternative Combination Therapy for PDL1 Negative Metastatic Cervical Cancer
- PMID: 32964058
- PMCID: PMC7492938
- DOI: 10.1155/2020/8345235
TILs and Anti-PD1 Therapy: An Alternative Combination Therapy for PDL1 Negative Metastatic Cervical Cancer
Abstract
Background: We investigated the efficacy of TILs and anti-PD1 combination therapy in patients with metastatic cervical cancer with low MSI expression and PDL1-negative.
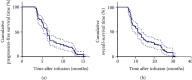
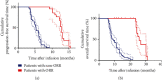
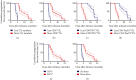
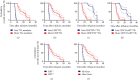
Methods: A total of 80 patients were put on TILs and anti-PD1 combination therapy, and the progression-free survival time (PFS) and overall survival time (OS) were assessed by Kaplan-Meier analysis. Univariate and multivariate analyses were performed to identify factors that could predict the prognosis of metastatic cervical cancer in the previously described patients.
Results: The objective response rate was 25%, whereas the mPFS and mOS were 6.1 and 11.3 months, respectively. The therapeutic efficacy was influenced by the characteristics of TILs, infection with HPV, and development of fever just after the therapy.
Conclusion: Overall, our results show that the combination therapy of TILs and anti-PD1 significantly improves the prognosis of metastatic cervical cancer.
Copyright © 2020 Huanhuan Yin et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
PD-L1 expression is associated with tumor infiltrating lymphocytes that predict response to NACT in squamous cell cervical cancer.Virchows Arch. 2021 Mar;478(3):517-525. doi: 10.1007/s00428-020-02922-5. Epub 2020 Sep 11. Virchows Arch. 2021. PMID: 32915266
-
PD‑L1/PD‑1 blockade in breast cancer: The immunotherapy era (Review).Oncol Rep. 2021 Jan;45(1):5-12. doi: 10.3892/or.2020.7831. Epub 2020 Nov 3. Oncol Rep. 2021. PMID: 33416128 Review.
-
Anti-4-1BB×PDL1 Bispecific Antibody Reinvigorates Tumor-Specific Exhausted CD8+ T Cells and Enhances the Efficacy of Anti-PD1 Blockade.Clin Cancer Res. 2024 Sep 13;30(18):4155-4166. doi: 10.1158/1078-0432.CCR-23-2864. Clin Cancer Res. 2024. PMID: 38743752
-
PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer.Cancer Biol Ther. 2018 May 4;19(5):373-380. doi: 10.1080/15384047.2018.1423919. Epub 2018 Feb 16. Cancer Biol Ther. 2018. PMID: 29336717 Free PMC article.
-
A combination of PD‑1/PD‑L1 inhibitors: The prospect of overcoming the weakness of tumor immunotherapy (Review).Mol Med Rep. 2021 May;23(5):362. doi: 10.3892/mmr.2021.12001. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760188 Free PMC article. Review.
Cited by
-
MicroRNA-300 Inhibits the Proliferation and Metastasis of Cervical Cancer Cells via Posttranscriptional Suppression of G Protein-Coupled Receptor 34 (GPR34).J Oncol. 2021 Dec 14;2021:2669822. doi: 10.1155/2021/2669822. eCollection 2021. J Oncol. 2021. PMID: 34950207 Free PMC article.
-
Emerging Trends in Immunotherapy for Cancer.Diseases. 2022 Sep 6;10(3):60. doi: 10.3390/diseases10030060. Diseases. 2022. PMID: 36135216 Free PMC article. Review.
-
Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors.Cancers (Basel). 2023 Feb 10;15(4):1137. doi: 10.3390/cancers15041137. Cancers (Basel). 2023. PMID: 36831480 Free PMC article. Review.
-
Immune checkpoint inhibitors in cervical cancer: Current status and research progress.Front Oncol. 2022 Oct 27;12:984896. doi: 10.3389/fonc.2022.984896. eCollection 2022. Front Oncol. 2022. PMID: 36387196 Free PMC article. Review.
-
Immune checkpoint blockade for locally advanced or recurrent/metastatic cervical cancer: An update on clinical data.Front Oncol. 2022 Dec 22;12:1045481. doi: 10.3389/fonc.2022.1045481. eCollection 2022. Front Oncol. 2022. PMID: 36644634 Free PMC article. Review.
References
-
- Kamangar F., Dores G. M., Anderson W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

