B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease
- PMID: 32961717
- PMCID: PMC7551072
- DOI: 10.3390/nu12092867
B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease
Abstract
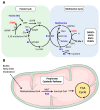
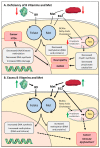
Vitamins B9 (folate) and B12 are essential water-soluble vitamins that play a crucial role in the maintenance of one-carbon metabolism: a set of interconnected biochemical pathways driven by folate and methionine to generate methyl groups for use in DNA synthesis, amino acid homeostasis, antioxidant generation, and epigenetic regulation. Dietary deficiencies in B9 and B12, or genetic polymorphisms that influence the activity of enzymes involved in the folate or methionine cycles, are known to cause developmental defects, impair cognitive function, or block normal blood production. Nutritional deficiencies have historically been treated with dietary supplementation or high-dose parenteral administration that can reverse symptoms in the majority of cases. Elevated levels of these vitamins have more recently been shown to correlate with immune dysfunction, cancer, and increased mortality. Therapies that specifically target one-carbon metabolism are therefore currently being explored for the treatment of immune disorders and cancer. In this review, we will highlight recent studies aimed at elucidating the role of folate, B12, and methionine in one-carbon metabolism during normal cellular processes and in the context of disease progression.
Keywords: Vitamin B12; folate; methionine; one-carbon metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Different dietary combinations of folic acid and vitamin B12 in parental diet results in epigenetic reprogramming of IGF2R and KCNQ1OT1 in placenta and fetal tissues in mice.Mol Reprod Dev. 2021 Jun;88(6):437-458. doi: 10.1002/mrd.23477. Epub 2021 May 18. Mol Reprod Dev. 2021. PMID: 34008284
-
Vitamin B12 , folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation.J Inherit Metab Dis. 2019 Jul;42(4):673-685. doi: 10.1002/jimd.12009. Epub 2019 Jan 28. J Inherit Metab Dis. 2019. PMID: 30693532 Review.
-
Effect of imbalance in folate and vitamin B12 in maternal/parental diet on global methylation and regulatory miRNAs.Sci Rep. 2019 Nov 26;9(1):17602. doi: 10.1038/s41598-019-54070-9. Sci Rep. 2019. PMID: 31772242 Free PMC article.
-
Folate, vitamin B12 and vitamin B6 and one carbon metabolism.J Nutr Health Aging. 2002;6(1):39-42. J Nutr Health Aging. 2002. PMID: 11813080 Review.
-
Folic acid with or without vitamin B12 for cognition and dementia.Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2. PMID: 14584018 Updated. Review.
Cited by
-
Reproductive-dependent effects of B vitamin deficiency on lifespan and physiology.Front Nutr. 2023 Oct 24;10:1277715. doi: 10.3389/fnut.2023.1277715. eCollection 2023. Front Nutr. 2023. PMID: 37941770 Free PMC article.
-
Folate and vitamin B12 usual intake and biomarker status by intake source in United States adults aged ≥19 y: NHANES 2007-2018.Am J Clin Nutr. 2023 Jul;118(1):241-254. doi: 10.1016/j.ajcnut.2023.05.016. Epub 2023 May 11. Am J Clin Nutr. 2023. PMID: 37172826 Free PMC article.
-
Vitamin B12-Multifaceted In Vivo Functions and In Vitro Applications.Nutrients. 2023 Jun 13;15(12):2734. doi: 10.3390/nu15122734. Nutrients. 2023. PMID: 37375638 Free PMC article. Review.
-
Impact of increasing one-carbon metabolites on traumatic brain injury outcome using pre-clinical models.Neural Regen Res. 2024 Aug 1;19(8):1728-1733. doi: 10.4103/1673-5374.389629. Epub 2023 Dec 11. Neural Regen Res. 2024. PMID: 38103238 Free PMC article.
-
Exploring the complexities of 1C metabolism: implications in aging and neurodegenerative diseases.Front Aging Neurosci. 2024 Jan 4;15:1322419. doi: 10.3389/fnagi.2023.1322419. eCollection 2023. Front Aging Neurosci. 2024. PMID: 38239489 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

