Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults
- PMID: 32958495
- PMCID: PMC7507250
- DOI: 10.1136/bmjopen-2020-041437
Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults
Abstract
Introduction: To find effective and safe treatments for COVID-19, the WHO recommended to systemically evaluate experimental therapeutics in collaborative randomised clinical trials. As COVID-19 was spreading in Europe, the French national institute for Health and Medical Research (Inserm) established a transdisciplinary team to develop a multi-arm randomised controlled trial named DisCoVeRy. The objective of the trial is to evaluate the clinical efficacy and safety of different investigational re-purposed therapeutics relative to Standard of Care (SoC) in patients hospitalised with COVID-19.
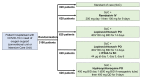
Methods and analysis: DisCoVeRy is a phase III, open-label, adaptive, controlled, multicentre clinical trial in which hospitalised patients with COVID-19 in need of oxygen therapy are randomised between five arms: (1) a control group managed with SoC and four therapeutic arms with re-purposed antiviral agents: (2) remdesivir + SoC, (3) lopinavir/ritonavir + SoC, (4) lopinavir/ritonavir associated with interferon (IFN)-β-1a + SoC and (5) hydroxychloroquine + SoC. The primary endpoint is the clinical status at Day 15 on the 7-point ordinal scale of the WHO Master Protocol (V.3.0, 3 March 2020). This trial involves patients hospitalised in conventional departments or intensive care units both from academic or non-academic hospitals throughout Europe. A sample size of 3100 patients (620 patients per arm) is targeted. This trial has begun on 22 March 2020. Since 5 April 2020, DisCoVeRy has been an add-on trial of the Solidarity consortium of trials conducted by the WHO in Europe and worldwide. On 8 June 2020, 754 patients have been included.
Ethics and dissemination: Inserm is the sponsor of DisCoVeRy. Ethical approval has been obtained from the institutional review board on 13 March 2020 (20.03.06.51744) and from the French National Agency for Medicines and Health Products (ANSM) on 9 March 2020. Results will be submitted for publication in peer-reviewed journals.
Trial registration number: NCT04315948 Eudra-CT 2020-000936-23.
Keywords: clinical trials; infectious diseases; intensive & critical care; public health; respiratory infections.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: François-Xavier Lescure reports fees for development of educational presentations from Gilead, outside the submitted work. Dominique Costagliola reports personal fees from Merck Switzerland, grants and personal fees from MSD France, personal fees from Gilead France, grants and personal fees from Janssen, outside the submitted work. Jean-François Timsit reports grants and personal fees from Merck, grants and personal fees from Pfizer, grants from biomerieux, personal fees from medimune, personal fees from Paratek, personal fees from Gilead, outside the submitted work. Benjamin Hamze reports personal fees from Sanofi, outside the submitted work. Gilles Peytavin has received travel grants, consultancy fees, honoraria, or study grants from various pharmaceutical companies, including Gilead Sciences, Merck, TheraTechnologies and ViiV Healthcare. France Mentre reports grants and personal fees from Sanofi, outside the submitted work.
Figures
Similar articles
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial.Lancet Infect Dis. 2022 Feb;22(2):209-221. doi: 10.1016/S1473-3099(21)00485-0. Epub 2021 Sep 14. Lancet Infect Dis. 2022. PMID: 34534511 Free PMC article. Clinical Trial.
-
An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a (the base therapeutic regimen) in moderate to severe COVID-19: A structured summary of a study protocol for a randomized controlled l trial.Trials. 2020 Oct 26;21(1):880. doi: 10.1186/s13063-020-04812-2. Trials. 2020. PMID: 33106183 Free PMC article. Clinical Trial.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
-
Potential therapeutic agents against COVID-19: What we know so far.J Chin Med Assoc. 2020 Jun;83(6):534-536. doi: 10.1097/JCMA.0000000000000318. J Chin Med Assoc. 2020. PMID: 32243270 Free PMC article. Review.
Cited by
-
Distributive randomization: a pragmatic fractional factorial design to screen or evaluate multiple simultaneous interventions in a clinical trial.BMC Med Res Methodol. 2024 Mar 11;24(1):64. doi: 10.1186/s12874-024-02191-9. BMC Med Res Methodol. 2024. PMID: 38468221 Free PMC article.
-
Association between SARS-CoV-2 viral kinetics and clinical score evolution in hospitalized patients.CPT Pharmacometrics Syst Pharmacol. 2023 Dec;12(12):2027-2037. doi: 10.1002/psp4.13051. Epub 2023 Oct 11. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 37728045 Free PMC article. Clinical Trial.
-
Remdesivir.Hosp Pharm. 2023 Oct;58(5):420-430. doi: 10.1177/0018578721999804. Epub 2021 Mar 6. Hosp Pharm. 2023. PMID: 37711410 Free PMC article. Review.
-
Drug repurposing for COVID-19: current evidence from randomized controlled adaptive platform trials and living systematic reviews.Br Med Bull. 2023 Sep 12;147(1):31-49. doi: 10.1093/bmb/ldac037. Br Med Bull. 2023. PMID: 37312588 Free PMC article.
-
Management of pharmacovigilance during the COVID-19 pandemic crisis by the safety department of an academic sponsor: Lessons learnt and challenges from the EU DisCoVeRy clinical trial.Pharmacol Res Perspect. 2023 Jun;11(3):e01072. doi: 10.1002/prp2.1072. Pharmacol Res Perspect. 2023. PMID: 37269068 Free PMC article. Review.
References
-
- World Health Organization WHO characterizes COVID-19 as a pandemic, 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a... [Accessed 14 Mar 2020].
-
- World Health Organization WHO R&D blueprint novel coronavirus COVID-19 therapeutic trial synopsis, 2020. Available: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Trea... [Accessed 10 Mar 2020].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous