Targeting Receptors on Cancer Cells with Protein Toxins
- PMID: 32957689
- PMCID: PMC7563326
- DOI: 10.3390/biom10091331
Targeting Receptors on Cancer Cells with Protein Toxins
Abstract
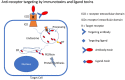
Cancer cells frequently upregulate surface receptors that promote growth and survival. These receptors constitute valid targets for intervention. One strategy involves the delivery of toxic payloads with the goal of killing those cancer cells with high receptor levels. Delivery can be accomplished by attaching a toxic payload to either a receptor-binding antibody or a receptor-binding ligand. Generally, the cell-binding domain of the toxin is replaced with a ligand or antibody that dictates a new binding specificity. The advantage of this "immunotoxin" approach lies in the potency of these chimeric molecules for killing cancer cells. However, receptor expression on normal tissue represents a significant obstacle to therapeutic intervention.
Keywords: cancer; diphtheria; immunotoxin; pseudomonas; receptor; ricin; toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Potent and specific killing of human malignant brain tumor cells by an anti-transferrin receptor antibody-ricin immunotoxin.J Neurosurg. 1987 Jun;66(6):850-61. doi: 10.3171/jns.1987.66.6.0850. J Neurosurg. 1987. PMID: 3033171
-
Targeted toxin therapy for the treatment of cancer.J Natl Cancer Inst. 1989 Oct 4;81(19):1455-63. doi: 10.1093/jnci/81.19.1455. J Natl Cancer Inst. 1989. PMID: 2550658 Review.
-
Mutations in diphtheria toxin separate binding from entry and amplify immunotoxin selectivity.Science. 1987 Oct 23;238(4826):536-9. doi: 10.1126/science.3498987. Science. 1987. PMID: 3498987
-
Antibody internalization after cell surface antigen binding is critical for immunotoxin development.Bioconjug Chem. 2009 Oct 21;20(10):1975-82. doi: 10.1021/bc900333j. Epub 2009 Sep 28. Bioconjug Chem. 2009. PMID: 19785403
-
Immunotoxin: A new tool for cancer therapy.Tumour Biol. 2017 Feb;39(2):1010428317692226. doi: 10.1177/1010428317692226. Tumour Biol. 2017. PMID: 28218037 Review.
Cited by
-
Treatment of ovarian cancer with modified anthrax toxin.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2210179119. doi: 10.1073/pnas.2210179119. Epub 2022 Aug 2. Proc Natl Acad Sci U S A. 2022. PMID: 35917343 Free PMC article. No abstract available.
-
The Urokinase Receptor (uPAR) as a "Trojan Horse" in Targeted Cancer Therapy: Challenges and Opportunities.Cancers (Basel). 2021 Oct 27;13(21):5376. doi: 10.3390/cancers13215376. Cancers (Basel). 2021. PMID: 34771541 Free PMC article. Review.
-
"Smart" drug delivery: A window to future of translational medicine.Front Chem. 2023 Jan 4;10:1095598. doi: 10.3389/fchem.2022.1095598. eCollection 2022. Front Chem. 2023. PMID: 36688039 Free PMC article. Review.
-
Antitumor Efficacy of EGFR-Targeted Recombinant Immunotoxin in Human Head and Neck Squamous Cell Carcinoma.Biology (Basel). 2022 Mar 22;11(4):486. doi: 10.3390/biology11040486. Biology (Basel). 2022. PMID: 35453686 Free PMC article.
-
Cytosolic Protein Delivery Using Modular Biotin-Streptavidin Assembly of Nanocomposites.ACS Nano. 2022 May 24;16(5):7323-7330. doi: 10.1021/acsnano.1c06768. Epub 2022 Apr 18. ACS Nano. 2022. PMID: 35435664 Free PMC article.
References
-
- Vitetta E.S., Thorpe P.E. Immunotoxins containing ricin or its a chain. Semin. Cell Biol. 1991;2:47–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

