Novel BAG3 Variants in African American Patients With Cardiomyopathy: Reduced β-Adrenergic Responsiveness in Excitation-Contraction
- PMID: 32956817
- PMCID: PMC7736114
- DOI: 10.1016/j.cardfail.2020.09.009
Novel BAG3 Variants in African American Patients With Cardiomyopathy: Reduced β-Adrenergic Responsiveness in Excitation-Contraction
Abstract
Background: We reported 3 novel nonsynonymous single nucleotide variants of Bcl2-associated athanogene 3 (BAG3) in African Americans with heart failure (HF) that are associated with a 2-fold increase in cardiac events (HF hospitalization, heart transplantation, or death).
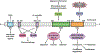
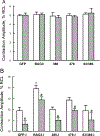
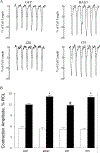
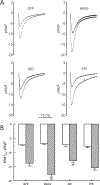
Methods and results: We expressed BAG3 variants (P63A, P380S, and A479V) via adenovirus-mediated gene transfer in adult left ventricular myocytes isolated from either wild-type (WT) or cardiac-specific BAG3 haploinsufficient (cBAG3+/-) mice: the latter to simulate the clinical situation in which BAG3 variants are only found on 1 allele. Compared with WT myocytes, cBAG3+/- myocytes expressed approximately 50% of endogenous BAG3 levels and exhibited decreased [Ca2+]i and contraction amplitudes after isoproterenol owing to decreased L-type Ca2+ current. BAG3 repletion with WT BAG3 but not P380S, A479V, or P63A/P380S variants restored contraction amplitudes in cBAG3+/- myocytes to those measured in WT myocytes, suggesting excitation-contraction abnormalities partly account for HF in patients harboring these mutants. Because P63A is near the WW domain (residues 21-55) and A479V is in the BAG domain (residues 420-499), we expressed BAG3 deletion mutants (Δ1-61 and Δ421-575) in WT myocytes and demonstrated that the BAG but not the WW domain was involved in enhancement of excitation-contraction by isoproterenol.
Conclusions: The BAG3 variants contribute to HF in African American patients partly by decreasing myocyte excitation-contraction under stress, and that both the BAG and PXXP domains are involved in mediating β-adrenergic responsiveness in myocytes.
Keywords: BAG3; adenovirus-mediated gene transfer; dilated cardiomyopathy; excitation–contraction coupling; isolated adult cardiac myocytes.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest K.K. is a board member, scientific advisor, and holds equity in Excision Biotherapeutics, a biotech start-up that has licensed the viral gene editing technology from Temple University for commercial development and clinical trials. A.M.F. and K.K. have a pending US patent #611934,483 for BAG3 as a target for heart failure therapy. A.M.F. and J.Y.C. have a pending US patent #621205,990 for BAG3 composition and methods. Exclusive rights to the patents have been optioned by Temple University to Renovacor, Inc. A.M.F. and J.Y.C. hold equity in Renovacor, Inc.
Figures



Similar articles
-
Genetics of BAG3: A Paradigm for Developing Precision Therapies for Dilated Cardiomyopathies.J Am Heart Assoc. 2022 Dec 6;11(23):e027373. doi: 10.1161/JAHA.122.027373. Epub 2022 Nov 16. J Am Heart Assoc. 2022. PMID: 36382946 Free PMC article. Review.
-
BAG3 regulates contractility and Ca(2+) homeostasis in adult mouse ventricular myocytes.J Mol Cell Cardiol. 2016 Mar;92:10-20. doi: 10.1016/j.yjmcc.2016.01.015. Epub 2016 Jan 19. J Mol Cell Cardiol. 2016. PMID: 26796036 Free PMC article.
-
Haplo-insufficiency of Bcl2-associated athanogene 3 in mice results in progressive left ventricular dysfunction, β-adrenergic insensitivity, and increased apoptosis.J Cell Physiol. 2018 Sep;233(9):6319-6326. doi: 10.1002/jcp.26482. Epub 2018 Mar 30. J Cell Physiol. 2018. PMID: 29323723 Free PMC article.
-
Mitochondrial dysfunction in human immunodeficiency virus-1 transgenic mouse cardiac myocytes.J Cell Physiol. 2019 Apr;234(4):4432-4444. doi: 10.1002/jcp.27232. Epub 2018 Sep 7. J Cell Physiol. 2019. PMID: 30256393 Free PMC article.
-
Advances in the role and mechanism of BAG3 in dilated cardiomyopathy.Heart Fail Rev. 2021 Jan;26(1):183-194. doi: 10.1007/s10741-019-09899-7. Heart Fail Rev. 2021. PMID: 31808029 Review.
Cited by
-
Genetics of BAG3: A Paradigm for Developing Precision Therapies for Dilated Cardiomyopathies.J Am Heart Assoc. 2022 Dec 6;11(23):e027373. doi: 10.1161/JAHA.122.027373. Epub 2022 Nov 16. J Am Heart Assoc. 2022. PMID: 36382946 Free PMC article. Review.
-
BAG3: Nature's Quintessential Multi-Functional Protein Functions as a Ubiquitous Intra-Cellular Glue.Cells. 2023 Mar 19;12(6):937. doi: 10.3390/cells12060937. Cells. 2023. PMID: 36980278 Free PMC article. Review.
-
The genetic architecture of pediatric cardiomyopathy.Am J Hum Genet. 2022 Feb 3;109(2):282-298. doi: 10.1016/j.ajhg.2021.12.006. Epub 2022 Jan 12. Am J Hum Genet. 2022. PMID: 35026164 Free PMC article.
-
Dysregulated Autophagy and Sarcomere Dysfunction in Patients With Heart Failure With Co-Occurrence of P63A and P380S BAG3 Variants.J Am Heart Assoc. 2023 Dec 19;12(24):e029938. doi: 10.1161/JAHA.123.029938. Epub 2023 Dec 18. J Am Heart Assoc. 2023. PMID: 38108245 Free PMC article.
References
-
- Myers VD, Tomar D, Madesh M, Wang J, Song J, Zhang XQ, Gupta MK, Tahrir FG, Gordon J, McClung JM, Kontos CD, Khalili K, Cheung JY and Feldman AM. Haplo-insufficiency of Bcl2-associated athanogene 3 in mice results in progressive left ventricular dysfunction, β-adrenergic insensitivity, and increased apoptosis. Journal of cellular physiology. 2018;233:6319–6326. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

