Lipophilicity trends upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl groups
- PMID: 32952731
- PMCID: PMC7476584
- DOI: 10.3762/bjoc.16.182
Lipophilicity trends upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl groups
Abstract
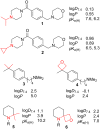
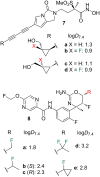
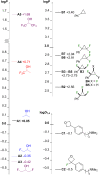
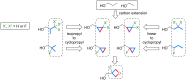
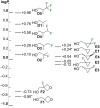
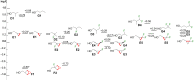
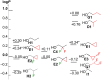
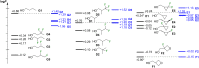
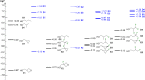
A systematic comparison of lipophilicity modulations upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl substituents, at a single carbon atom, is provided using directly comparable, and easily accessible model compounds. In addition, comparison with relevant linear chain derivatives is provided, as well as lipophilicity changes occurring upon chain extension of acyclic precursors to give cyclopropyl containing compounds. For the compounds investigated, fluorination of the isopropyl substituent led to larger lipophilicity modulation compared to fluorination of the cyclopropyl substituent.
Keywords: aliphatic fluorination; cyclopropane; isopropyl; isostere; lipophilicity; oxetane.
Copyright © 2020, Jeffries et al.; licensee Beilstein-Institut.
Figures


Similar articles
-
Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs.J Med Chem. 2020 Feb 13;63(3):1002-1031. doi: 10.1021/acs.jmedchem.9b01172. Epub 2020 Jan 17. J Med Chem. 2020. PMID: 31894985
-
Skipped Fluorination Motifs: Synthesis of Building Blocks and Comparison of Lipophilicity Trends with Vicinal and Isolated Fluorination Motifs.J Org Chem. 2021 Jan 15;86(2):1882-1900. doi: 10.1021/acs.joc.0c02810. Epub 2021 Jan 5. J Org Chem. 2021. PMID: 33400526
-
Investigating the Influence of (Deoxy)fluorination on the Lipophilicity of Non-UV-Active Fluorinated Alkanols and Carbohydrates by a New log P Determination Method.Angew Chem Int Ed Engl. 2016 Jan 11;55(2):674-8. doi: 10.1002/anie.201509460. Epub 2015 Nov 23. Angew Chem Int Ed Engl. 2016. PMID: 26592706 Free PMC article.
-
Fluorination methods in drug discovery.Org Biomol Chem. 2016 Sep 28;14(36):8398-427. doi: 10.1039/c6ob00764c. Epub 2016 Aug 10. Org Biomol Chem. 2016. PMID: 27506398 Review.
-
Most stable conformation of the cyclopropane ring attached at a carbon atom in a 1,2-dicarba-closo-dodecaborane(12) system.J Org Chem. 2004 Jun 11;69(12):4063-74. doi: 10.1021/jo049854i. J Org Chem. 2004. PMID: 15176831
Cited by
-
Lipophilicity Modulations by Fluorination Correlate with Membrane Partitioning.Angew Chem Int Ed Engl. 2023 May 15;62(21):e202301077. doi: 10.1002/anie.202301077. Epub 2023 Apr 17. Angew Chem Int Ed Engl. 2023. PMID: 36932824 Free PMC article.
-
Synthesis and Physicochemical Properties of 2-SF5-(Aza)Indoles, a New Family of SF5 Heterocycles.ACS Org Inorg Au. 2021 Jul 6;1(2):43-50. doi: 10.1021/acsorginorgau.1c00010. eCollection 2021 Dec 1. ACS Org Inorg Au. 2021. PMID: 36855754 Free PMC article.
-
29th Annual GP2A Medicinal Chemistry Conference.Pharmaceuticals (Basel). 2021 Dec 7;14(12):1278. doi: 10.3390/ph14121278. Pharmaceuticals (Basel). 2021. PMID: 34959677 Free PMC article.
-
Development of Isopropyl-Tailed Chalcones as a New Class of Selective MAO-B Inhibitors for the Treatment of Parkinson's Disorder.ACS Omega. 2023 Feb 7;8(7):6908-6917. doi: 10.1021/acsomega.2c07694. eCollection 2023 Feb 21. ACS Omega. 2023. PMID: 36844523 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
