Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D
- PMID: 32945351
- PMCID: PMC7447310
- DOI: 10.3892/ijmm.2020.4692
Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D
Abstract
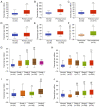
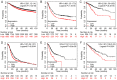
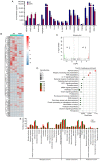
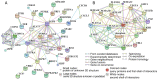
Angiogenesis and vascular maturation play important roles in tumorigenesis and tumor development. The expression of neuropilin 1 (NRP1) is closely associated with angiogenesis in tumors; however, the molecular mechanisms of action in angiogenesis and tumor maturation, as well as the potential clinical value of NRP1 remain unclear. The importance of NRP1 expression in tumor progression was determined using The Cancer Genome Atlas (TCGA) database analysis. Gain‑ and loss‑of‑function experiments of NRP1 were performed in vascular endothelial cells (ECs) to investigate the functions in angiogenesis. CCK‑8, flow cytometry, Transwell experiments and a series of in vitro experiments were used to detect cell functions. A combination of angiogenesis antibody arrays and RNA‑Seq analyses were performed to reveal the proangiogenic mechanisms of action. The function of semaphorin 4D (SEMA4D) was also investigated separately. NRP1 mRNA levels were significantly increased in primary tumors compared with normal tissues based on TCGA data (P<0.01) and were associated with tumor development in patients. Gain‑ and loss‑of‑function experiments highlighted the function of NRP1 in promoting EC proliferation, motility and capillary‑like tube formation and in reducing apoptosis. NRP1 overexpression led to significantly decreased EC markers (PECAM‑1, angiogenin, PIGF and MMP‑9) expression levels and reduced the vascular maturity. MAPK7, TPM1, RRBP1, PTPRK, HSP90A, PRKD2, PFKFB3, RGS4 and SPARC were revealed to play important roles in this process. SEMA4D was revealed to be a key protein associated with NRP1 in ECs. These data indicated that NRP1‑promoted angiogenesis may be induced at the cost of reducing maturity of the ECs. NRP1 may also be a therapeutic target for antiangiogenic strategies and a candidate prognostic marker for tumors.
Figures

Similar articles
-
Loss of semaphorin-neuropilin-1 signaling causes dysmorphic vascularization reminiscent of alveolar capillary dysplasia.Am J Pathol. 2012 Dec;181(6):2003-17. doi: 10.1016/j.ajpath.2012.08.037. Epub 2012 Oct 12. Am J Pathol. 2012. PMID: 23063659
-
Neuropilin-1 enforces extracellular matrix signalling via ABL1 to promote angiogenesis.Biochem Soc Trans. 2014 Oct;42(5):1429-34. doi: 10.1042/BST20140141. Biochem Soc Trans. 2014. PMID: 25233427 Review.
-
Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis.Ann Rheum Dis. 2016 Aug;75(8):1541-9. doi: 10.1136/annrheumdis-2015-207483. Epub 2015 Sep 10. Ann Rheum Dis. 2016. PMID: 26359450
-
Plexin-B1 activates NF-κB and IL-8 to promote a pro-angiogenic response in endothelial cells.PLoS One. 2011;6(10):e25826. doi: 10.1371/journal.pone.0025826. Epub 2011 Oct 18. PLoS One. 2011. Retraction in: PLoS One. 2019 Sep 26;14(9):e0223295. doi: 10.1371/journal.pone.0223295. PMID: 22028792 Free PMC article. Retracted.
-
Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability.Microcirculation. 2014 May;21(4):315-23. doi: 10.1111/micc.12124. Microcirculation. 2014. PMID: 24521511 Free PMC article. Review.
Cited by
-
HIF-1α promoted vasculogenic mimicry formation in lung adenocarcinoma through NRP1 upregulation in the hypoxic tumor microenvironment.Cell Death Dis. 2021 Apr 13;12(4):394. doi: 10.1038/s41419-021-03682-z. Cell Death Dis. 2021. PMID: 33850110 Free PMC article.
-
Yolk sac cell atlas reveals multiorgan functions during human early development.Science. 2023 Aug 18;381(6659):eadd7564. doi: 10.1126/science.add7564. Epub 2023 Aug 18. Science. 2023. PMID: 37590359 Free PMC article.
-
Proteomic Signature of Subclinical Coronary Artery Disease in People With HIV: Analysis of the REPRIEVE Mechanistic Substudy.J Infect Dis. 2022 Nov 11;226(10):1809-1822. doi: 10.1093/infdis/jiac196. J Infect Dis. 2022. PMID: 35535576 Free PMC article.
-
An Essential NRP1-Mediated Role for Tagln2 in Gastric Cancer Angiogenesis.Front Oncol. 2021 Jun 4;11:653246. doi: 10.3389/fonc.2021.653246. eCollection 2021. Front Oncol. 2021. PMID: 34150622 Free PMC article.
-
New Biosensor for Determination of Neuropilin-1 with Detection by Surface Plasmon Resonance Imaging.Sensors (Basel). 2023 Apr 19;23(8):4118. doi: 10.3390/s23084118. Sensors (Basel). 2023. PMID: 37112459 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

