Proteasomal and lysosomal degradation for specific and durable suppression of immunotherapeutic targets
- PMID: 32944392
- PMCID: PMC7476092
- DOI: 10.20892/j.issn.2095-3941.2020.0066
Proteasomal and lysosomal degradation for specific and durable suppression of immunotherapeutic targets
Abstract
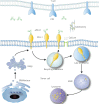
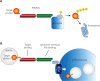
Cancer immunotherapy harness the body's immune system to eliminate cancer, by using a broad panel of soluble and membrane proteins as therapeutic targets. Immunosuppression signaling mediated by ligand-receptor interaction may be blocked by monoclonal antibodies, but because of repopulation of the membrane via intracellular organelles, targets must be eliminated in whole cells. Targeted protein degradation, as exemplified in proteolysis targeting chimera (PROTAC) studies, is a promising strategy for selective inhibition of target proteins. The recently reported use of lysosomal targeting molecules to eliminate immune checkpoint proteins has paved the way for targeted degradation of membrane proteins as crucial anti-cancer targets. Further studies on these molecules' modes of action, target-binding "warheads", lysosomal sorting signals, and linker design should facilitate their rational design. Modifications and derivatives may improve their cell-penetrating ability and the in vivo stability of these pro-drugs. These studies suggest the promise of alternative strategies for cancer immunotherapy, with the aim of achieving more potent and durable suppression of tumor growth. Here, the successes and limitations of antibody inhibitors in cancer immunotherapy, as well as research progress on PROTAC- and lysosomal-dependent degradation of target proteins, are reviewed.
Keywords: Cancer immunotherapy; PROTAC; membrane protein; targeted degradation.
Copyright: © 2020, Cancer Biology & Medicine.
Figures
Similar articles
-
Targeted degradation of immune checkpoint proteins: emerging strategies for cancer immunotherapy.Oncogene. 2020 Nov;39(48):7106-7113. doi: 10.1038/s41388-020-01491-w. Epub 2020 Oct 6. Oncogene. 2020. PMID: 33024277 Review.
-
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy.J Hematol Oncol. 2020 May 13;13(1):50. doi: 10.1186/s13045-020-00885-3. J Hematol Oncol. 2020. PMID: 32404196 Free PMC article. Review.
-
[Protein induced proximity and targeted degradations by new degraders: concepts, developments, challenges for clinical applications].Biol Aujourdhui. 2024;218(1-2):41-54. doi: 10.1051/jbio/2024007. Epub 2024 Jul 15. Biol Aujourdhui. 2024. PMID: 39007776 Review. French.
-
Targeted Protein Degradation: An Emerging Therapeutic Strategy in Cancer.Anticancer Agents Med Chem. 2021;21(2):214-230. doi: 10.2174/1871520620666200410082652. Anticancer Agents Med Chem. 2021. PMID: 32275492 Review.
-
Effective degradation of EGFRL858R+T790M mutant proteins by CRBN-based PROTACs through both proteosome and autophagy/lysosome degradation systems.Eur J Med Chem. 2021 Jun 5;218:113328. doi: 10.1016/j.ejmech.2021.113328. Epub 2021 Mar 7. Eur J Med Chem. 2021. PMID: 33773286
Cited by
-
Targeted degradation of immune checkpoint proteins: emerging strategies for cancer immunotherapy.Oncogene. 2020 Nov;39(48):7106-7113. doi: 10.1038/s41388-020-01491-w. Epub 2020 Oct 6. Oncogene. 2020. PMID: 33024277 Review.
-
Small-Molecule PROTACs for Cancer Immunotherapy.Molecules. 2022 Aug 25;27(17):5439. doi: 10.3390/molecules27175439. Molecules. 2022. PMID: 36080223 Free PMC article. Review.
-
Update on Autophagy Inhibitors in Cancer: Opening up to a Therapeutic Combination with Immune Checkpoint Inhibitors.Cells. 2023 Jun 23;12(13):1702. doi: 10.3390/cells12131702. Cells. 2023. PMID: 37443736 Free PMC article. Review.
-
Endolysosomal transient receptor potential mucolipins and two-pore channels: implications for cancer immunity.Front Immunol. 2024 May 22;15:1389194. doi: 10.3389/fimmu.2024.1389194. eCollection 2024. Front Immunol. 2024. PMID: 38840905 Free PMC article. Review.
-
At the Cutting Edge against Cancer: A Perspective on Immunoproteasome and Immune Checkpoints Modulation as a Potential Therapeutic Intervention.Cancers (Basel). 2021 Sep 28;13(19):4852. doi: 10.3390/cancers13194852. Cancers (Basel). 2021. PMID: 34638337 Free PMC article. Review.
References
-
- Muir C, Menzies AM, Clifton-Bligh RJ, Tsang VHM. Thyroid toxicity following immune checkpoint inhibitor treatment in advanced cancer. Thyroid. 2020 Doi: 10.1089/thy.2020.0032. In press. - PubMed
-
- Park JG, Lee CR, Kim MG, Kim G, Shin HM, Jeon YH, et al. Kidney residency of VISTA-positive macrophages accelerates repair from ischemic injury. Kidney Int. 2019;97:980–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
