BCAT1 binds the RNA-binding protein ZNF423 to activate autophagy via the IRE1-XBP-1-RIDD axis in hypoxic PASMCs
- PMID: 32938905
- PMCID: PMC7494854
- DOI: 10.1038/s41419-020-02930-y
BCAT1 binds the RNA-binding protein ZNF423 to activate autophagy via the IRE1-XBP-1-RIDD axis in hypoxic PASMCs
Erratum in
-
Correction: BCAT1 binds the RNA-binding protein ZNF423 to activate autophagy via the IRE1-XBP-1-RIDD axis in hypoxic PASMCs.Cell Death Dis. 2021 Dec 23;13(1):27. doi: 10.1038/s41419-021-04466-1. Cell Death Dis. 2021. PMID: 34949759 Free PMC article. No abstract available.
Abstract
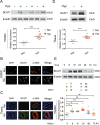
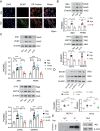
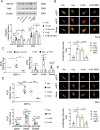
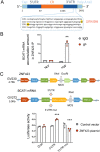
Abnormal functional changes in pulmonary artery smooth muscle cells are the main causes of many lung diseases. Among, autophagy plays a crucial role. However, the specific molecular regulatory mechanism of autophagy in PASMCs remains unclear. Here, we first demonstrate that BCAT1 played a key role in the autophagy of hypoxic PASMCs and hypoxic model rats. BCAT1-induced activation and accumulation of the autophagy signaling proteins BECN1 and Atg5 by the endoplasmic reticulum (ER) stress pathway. Interestingly, we discovered that BCAT1 bound IRE1 on the ER to activate expression of its downstream pathway XBP-1-RIDD axis to activate autophagy. More importantly, we identified an RNA-binding protein, zinc finger protein 423, which promoted autophagy by binding adenylate/uridylate (AU)-rich elements in the BCAT1 mRNA 3'-untranslated region. Overall, our results identify BCAT1 as a potential therapeutic target for the clinical treatment of lung diseases and reveal a novel posttranscriptional regulatory mechanism and signaling pathway in hypoxia-induced PASMC autophagy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Correction: BCAT1 binds the RNA-binding protein ZNF423 to activate autophagy via the IRE1-XBP-1-RIDD axis in hypoxic PASMCs.Cell Death Dis. 2021 Dec 23;13(1):27. doi: 10.1038/s41419-021-04466-1. Cell Death Dis. 2021. PMID: 34949759 Free PMC article. No abstract available.
-
Hypoxia induces pulmonary artery smooth muscle dysfunction through mitochondrial fragmentation-mediated endoplasmic reticulum stress.Aging (Albany NY). 2020 Nov 18;12(23):23684-23697. doi: 10.18632/aging.103892. Epub 2020 Nov 18. Aging (Albany NY). 2020. PMID: 33221740 Free PMC article.
-
The key role of PGC-1α in mitochondrial biogenesis and the proliferation of pulmonary artery vascular smooth muscle cells at an early stage of hypoxic exposure.Mol Cell Biochem. 2012 Aug;367(1-2):9-18. doi: 10.1007/s11010-012-1313-z. Epub 2012 Apr 15. Mol Cell Biochem. 2012. PMID: 22527940
-
Silencing of STIM1 attenuates hypoxia-induced PASMCs proliferation via inhibition of the SOC/Ca2+/NFAT pathway.Respir Res. 2013 Jan 5;14(1):2. doi: 10.1186/1465-9921-14-2. Respir Res. 2013. PMID: 23289723 Free PMC article.
-
Getting RIDD of RNA: IRE1 in cell fate regulation.Trends Biochem Sci. 2014 May;39(5):245-54. doi: 10.1016/j.tibs.2014.02.008. Epub 2014 Mar 20. Trends Biochem Sci. 2014. PMID: 24657016 Review.
Cited by
-
Research progress on branched-chain amino acid aminotransferases.Front Genet. 2023 Nov 6;14:1233669. doi: 10.3389/fgene.2023.1233669. eCollection 2023. Front Genet. 2023. PMID: 38028625 Free PMC article. Review.
-
The multifunctional RNA-binding protein Staufen1: an emerging regulator of oncogenesis through its various roles in key cellular events.Cell Mol Life Sci. 2021 Dec;78(23):7145-7160. doi: 10.1007/s00018-021-03965-w. Epub 2021 Oct 11. Cell Mol Life Sci. 2021. PMID: 34633481 Free PMC article. Review.
-
RNA-binding proteins and long noncoding RNAs in intestinal epithelial autophagy and barrier function.Tissue Barriers. 2021 Apr 3;9(2):1895648. doi: 10.1080/21688370.2021.1895648. Epub 2021 Mar 12. Tissue Barriers. 2021. PMID: 33709880 Free PMC article. Review.
-
FUNDC1-mediated mitophagy and HIF1α activation drives pulmonary hypertension during hypoxia.Cell Death Dis. 2022 Jul 21;13(7):634. doi: 10.1038/s41419-022-05091-2. Cell Death Dis. 2022. PMID: 35864106 Free PMC article.
-
Transcriptional and Epigenetic Factors Associated with Early Thrombosis of Femoral Artery Involved in Arteriovenous Fistula.Proteomes. 2022 Apr 30;10(2):14. doi: 10.3390/proteomes10020014. Proteomes. 2022. PMID: 35645372 Free PMC article.
References
-
- Elberson VD, Nielsen LC, Wang H, Kumar HS. Effects of intermittent hypoxia and hyperoxia on angiogenesis and lung development in newborn mice. J. Neonatal Perinat. Med. 2015;8:313–322. - PubMed
-
- Soriano JB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017;5:691–706. - PMC - PubMed
-
- Jin H, et al. Melatonin attenuates hypoxic pulmonary hypertension by inhibiting the inflammation and the proliferation of pulmonary arterial smooth muscle cells. J. Pineal Res. 2014;57:442–450. - PubMed
-
- Milara J, et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax. 2018;73:519–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

