T-cell responses and therapies against SARS-CoV-2 infection
- PMID: 32935333
- PMCID: PMC7730020
- DOI: 10.1111/imm.13262
T-cell responses and therapies against SARS-CoV-2 infection
Abstract
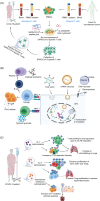
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2, a novel coronavirus strain. Some studies suggest that COVID-19 could be an immune-related disease, and failure of effective immune responses in initial stages of viral infection could contribute to systemic inflammation and tissue damage, leading to worse disease outcomes. T cells can act as a double-edge sword with both pro- and anti-roles in the progression of COVID-19. Thus, better understanding of their roles in immune responses to SARS-CoV-2 infection is crucial. T cells primarily react to the spike protein on the coronavirus to initiate antiviral immunity; however, T-cell responses can be suboptimal, impaired or excessive in severe COVID-19 patients. This review focuses on the multifaceted roles of T cells in COVID-19 pathogenesis and rationalizes their significance in eliciting appropriate antiviral immune responses in COVID-19 patients and unexposed individuals. In addition, we summarize the potential therapeutic approaches related to T cells to treat COVID-19 patients. These include adoptive T-cell therapies, vaccines activating T-cell responses, recombinant cytokines, Th1 activators and Th17 blockers, and potential utilization of immune checkpoint inhibitors alone or in combination with anti-inflammatory drugs to improve antiviral T-cell responses against SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; T cells; coronavirus; immune responses.
© 2020 The Authors. Immunology published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Innate immunity in COVID-19: Drivers of pathogenesis and potential therapeutic targets.Asian Pac J Allergy Immunol. 2021 Jun;39(2):69-77. doi: 10.12932/AP-130121-1037. Asian Pac J Allergy Immunol. 2021. PMID: 34174806 Review.
-
Emerging roles of extracellular vesicles in COVID-19, a double-edged sword?Immunology. 2021 Aug;163(4):416-430. doi: 10.1111/imm.13329. Epub 2021 May 4. Immunology. 2021. PMID: 33742451 Free PMC article. Review.
-
Phenotypes and Functions of SARS-CoV-2-Reactive T Cells.Mol Cells. 2021 Jun 30;44(6):401-407. doi: 10.14348/molcells.2021.0079. Mol Cells. 2021. PMID: 34120892 Free PMC article. Review.
-
Current state of vaccine development and targeted therapies for COVID-19: impact of basic science discoveries.Cardiovasc Pathol. 2021 Jan-Feb;50:107278. doi: 10.1016/j.carpath.2020.107278. Epub 2020 Sep 2. Cardiovasc Pathol. 2021. PMID: 32889088 Free PMC article. Review.
-
The Case for S2: The Potential Benefits of the S2 Subunit of the SARS-CoV-2 Spike Protein as an Immunogen in Fighting the COVID-19 Pandemic.Front Immunol. 2021 Mar 9;12:637651. doi: 10.3389/fimmu.2021.637651. eCollection 2021. Front Immunol. 2021. PMID: 33767706 Free PMC article.
Cited by
-
Therapeutic Role of Tocilizumab in SARS-CoV-2-Induced Cytokine Storm: Rationale and Current Evidence.Int J Mol Sci. 2021 Mar 17;22(6):3059. doi: 10.3390/ijms22063059. Int J Mol Sci. 2021. PMID: 33802761 Free PMC article. Review.
-
Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab.Vaccines (Basel). 2022 Dec 16;10(12):2167. doi: 10.3390/vaccines10122167. Vaccines (Basel). 2022. PMID: 36560576 Free PMC article.
-
Emerging toolset of three-dimensional pulmonary cell culture models for simulating lung pathophysiology towards mechanistic elucidation and therapeutic treatment of SARS-COV-2 infection.Front Pharmacol. 2022 Dec 12;13:1033043. doi: 10.3389/fphar.2022.1033043. eCollection 2022. Front Pharmacol. 2022. PMID: 36578545 Free PMC article. Review.
-
Regulatory T Cells as Predictors of Clinical Course in Hospitalised COVID-19 Patients.Front Immunol. 2021 Dec 2;12:789735. doi: 10.3389/fimmu.2021.789735. eCollection 2021. Front Immunol. 2021. PMID: 34925369 Free PMC article.
-
First-in-human immunoPET imaging of COVID-19 convalescent patients using dynamic total-body PET and a CD8-targeted minibody.Sci Adv. 2023 Oct 13;9(41):eadh7968. doi: 10.1126/sciadv.adh7968. Epub 2023 Oct 12. Sci Adv. 2023. PMID: 37824612 Free PMC article.
References
-
- World Health Organization . Coronavirus disease (COVID‐19) Pandemic 2020 Aug 22 [Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

