Potential relationship between Sirt3 and autophagy in ovarian cancer
- PMID: 32934730
- PMCID: PMC7471650
- DOI: 10.3892/ol.2020.12023
Potential relationship between Sirt3 and autophagy in ovarian cancer
Abstract
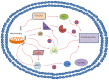
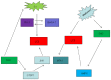
Sirtuin 3 (Sirt3) is an important member of the sirtuin protein family. It is a deacetylase that was previously reported to modulate the level of reactive oxygen species (ROS) production and limit the extent of oxidative damage in cellular components. As an important member of the class III type of histone deacetylases, Sirt3 has also been documented to mediate nuclear gene expression, metabolic control, neuroprotection, cell cycle and proliferation. In ovarian cancer (OC), Sirt3 has been reported to regulate cellular metabolism, apoptosis and autophagy. Sirt3 can regulate autophagy through a variety of different molecular signaling pathways, including the p62, 5'AMP-activated protein kinase and mitochondrial ROS-superoxide dismutase pathways. However, autophagy downstream of Sirt3 and its association with OC remains poorly understood. In the present review, the known characteristics of Sirt3 and autophagy were outlined, and their potential functional roles were discussed. Following a comprehensive analysis of the current literature, Sirt3 and autophagy may either serve positive or negative roles in the regulation of OC. Therefore, it is important to identify the appropriate expression level of Sirt3 to control the activation of autophagy in OC cells. This strategy may prove to be a novel therapeutic method to reduce the mortality of patients with OC. Finally, potential research directions into the association between Sirt3 and other signaling pathways were provided.
Keywords: autophagy; ovarian cancer; sirtuin 3.
Copyright: © Shi et al.
Figures
Similar articles
-
The novel relationship between Sirt3 and autophagy in myocardial ischemia-reperfusion.J Cell Physiol. 2019 May;234(5):5488-5495. doi: 10.1002/jcp.27329. Epub 2018 Nov 28. J Cell Physiol. 2019. PMID: 30485429 Review.
-
SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin.Autophagy. 2015;11(7):1037-51. doi: 10.1080/15548627.2015.1052208. Autophagy. 2015. PMID: 26120888 Free PMC article.
-
Autophagy maintains ubiquitination-proteasomal degradation of Sirt3 to limit oxidative stress in K562 leukemia cells.Oncotarget. 2016 Jun 14;7(24):35692-35702. doi: 10.18632/oncotarget.9592. Oncotarget. 2016. PMID: 27232755 Free PMC article.
-
Sirtuin Family Members Selectively Regulate Autophagy in Osteosarcoma and Mesothelioma Cells in Response to Cellular Stress.Front Oncol. 2019 Sep 24;9:949. doi: 10.3389/fonc.2019.00949. eCollection 2019. Front Oncol. 2019. PMID: 31608237 Free PMC article.
-
SIRT3: Oncogene and Tumor Suppressor in Cancer.Cancers (Basel). 2017 Jul 12;9(7):90. doi: 10.3390/cancers9070090. Cancers (Basel). 2017. PMID: 28704962 Free PMC article. Review.
Cited by
-
Advances in SIRT3 involvement in regulating autophagy-related mechanisms.Cell Div. 2024 Jun 12;19(1):20. doi: 10.1186/s13008-024-00124-y. Cell Div. 2024. PMID: 38867228 Free PMC article. Review.
-
Evaluation of ARK5 and SIRT3 expression in renal cell carcinoma and their clinical significance.Diagn Pathol. 2023 Nov 23;18(1):125. doi: 10.1186/s13000-023-01409-6. Diagn Pathol. 2023. PMID: 37996927 Free PMC article.
-
CRIF1 promotes the progression of non-small-cell lung cancer by SIRT3- mediated deacetylation of PYCR1.J Mol Histol. 2022 Aug;53(4):657-667. doi: 10.1007/s10735-022-10075-9. Epub 2022 Jun 18. J Mol Histol. 2022. PMID: 35716330
-
The role of protein acetylation in carcinogenesis and targeted drug discovery.Front Endocrinol (Lausanne). 2022 Sep 12;13:972312. doi: 10.3389/fendo.2022.972312. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36171897 Free PMC article. Review.
-
COVID-19 and cytokine storm syndrome: can what we know about interleukin-6 in ovarian cancer be applied?J Ovarian Res. 2021 Feb 8;14(1):28. doi: 10.1186/s13048-021-00772-6. J Ovarian Res. 2021. PMID: 33550983 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
