Mass Spectrometry and Structural Biology Techniques in the Studies on the Coronavirus-Receptor Interaction
- PMID: 32927621
- PMCID: PMC7571139
- DOI: 10.3390/molecules25184133
Mass Spectrometry and Structural Biology Techniques in the Studies on the Coronavirus-Receptor Interaction
Abstract
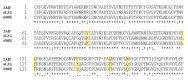
Mass spectrometry and some other biophysical methods, have made substantial contributions to the studies on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and human proteins interactions. The most interesting feature of SARS-CoV-2 seems to be the structure of its spike (S) protein and its interaction with the human cell receptor. Mass spectrometry of spike S protein revealed how the glycoforms are distributed across the S protein surface. X-ray crystallography and cryo-electron microscopy made huge impact on the studies on the S protein and ACE2 receptor protein interaction, by elucidating the three-dimensional structures of these proteins and their conformational changes. The findings of the most recent studies in the scope of SARS-CoV-2-Human protein-protein interactions are described here.
Keywords: MS; SARS coronavirus; glycosylation; spike protein-ACE2 interaction; structural techniques.
Conflict of interest statement
The author declares no conflict of interest.
Figures


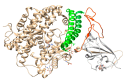
Similar articles
-
Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein.Virus Res. 2020 Sep;286:198058. doi: 10.1016/j.virusres.2020.198058. Epub 2020 Jun 9. Virus Res. 2020. PMID: 32531235 Free PMC article.
-
Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2.J Med Virol. 2020 Jun;92(6):595-601. doi: 10.1002/jmv.25726. Epub 2020 Mar 11. J Med Virol. 2020. PMID: 32100877 Free PMC article.
-
Structural basis of receptor recognition by SARS-CoV-2.Nature. 2020 May;581(7807):221-224. doi: 10.1038/s41586-020-2179-y. Epub 2020 Mar 30. Nature. 2020. PMID: 32225175 Free PMC article.
-
Molecular Basis of Pathogenesis of Coronaviruses: A Comparative Genomics Approach to Planetary Health to Prevent Zoonotic Outbreaks in the 21st Century.OMICS. 2020 Nov;24(11):634-644. doi: 10.1089/omi.2020.0131. Epub 2020 Sep 16. OMICS. 2020. PMID: 32940573 Review.
-
Angiotensin-converting enzyme 2: The old door for new severe acute respiratory syndrome coronavirus 2 infection.Rev Med Virol. 2020 Sep;30(5):e2122. doi: 10.1002/rmv.2122. Epub 2020 Jun 30. Rev Med Virol. 2020. PMID: 32602627 Free PMC article. Review.
Cited by
-
Thermodynamic analysis of the interactions between human ACE2 and spike RBD of Betacoronaviruses (SARS-CoV-1 and SARS-CoV-2).FEBS Open Bio. 2023 Jan;13(1):174-184. doi: 10.1002/2211-5463.13525. Epub 2022 Dec 9. FEBS Open Bio. 2023. PMID: 36416453 Free PMC article.
-
Perspectives and potential approaches for targeting neuropilin 1 in SARS-CoV-2 infection.Mol Med. 2021 Dec 27;27(1):162. doi: 10.1186/s10020-021-00423-y. Mol Med. 2021. PMID: 34961486 Free PMC article. Review.
-
N-glycosylation profiles of the SARS-CoV-2 spike D614G mutant and its ancestral protein characterized by advanced mass spectrometry.Sci Rep. 2021 Dec 7;11(1):23561. doi: 10.1038/s41598-021-02904-w. Sci Rep. 2021. PMID: 34876606 Free PMC article.
-
Development of a SARS-CoV-2-derived receptor-binding domain-based ACE2 biosensor.Sens Actuators B Chem. 2021 May 1;334:129663. doi: 10.1016/j.snb.2021.129663. Epub 2021 Feb 16. Sens Actuators B Chem. 2021. PMID: 33612970 Free PMC article.
-
Analytical Ultracentrifugation Detects Quaternary Rearrangements and Antibody-Induced Conformational Selection of the SARS-CoV-2 Spike Trimer.Int J Mol Sci. 2023 Oct 3;24(19):14875. doi: 10.3390/ijms241914875. Int J Mol Sci. 2023. PMID: 37834322 Free PMC article.
References
-
- Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M., Housa A., Almazroa M.A., Alraihan N., Banjar A., et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

